Do u you still need to distill canned butane that says 5-11 times distilled? If so how many additional times should I distill ? Also I’d like to start with cleanest gas possible before distilling … should I go with a major bulk gas supplier in the area or 5-11 times distilled cans ?
Doesn’t matter what the can says.
You shouldn’t use it.
Canned lighter fluid contains benzene, toluene & xylene which are carcinogenic.
Get 5 LB tank of N-butane
I still use distilled whip it premium…
Whip it. Yuck. That’s industrial / commercial grade butane run through a Mr. Coffee filter and a few activated carbon filters; like the ones that filter fish shit out of the water in my aquarium. That gets rid of the stink in the low purity cigarette lighter fluid, but it’s still low-purity cigarette lighter fluid.
Sadly, it still works as a solvent for botanical extraction. But I wouldn’t use it.
20 pound tanks of n-butane are readily available from many sources, some even offer actual high purity butane. I recommend you get a 20 pounder of good gas from one of them.
considering I don’t sell what I make, I think I’ll be okay
No reason to not buy tanks and distill it, distill it til you see nothing in your collection pot. Could be 1 time could be 3 times to bet all the gunk out.
Welcome to 2009. Wow. Is this real?
The quality gas supply companies for this market use very clean, dedicated equipment and cylinders. Canned butane is filled on an aerosol fill line with lower, aerosol grade gas and non-cleaned cans with whoknowswhat in them. So yes, if you must use canned butane, distill the shit out of it.
Literally…
How do you distill out benzene, toluene and xylene?
Well, the boiling point of benzene is 176.2f, toluene is 231.1f, and xylene is 282.2f. Pretty far of from butane at 30.2f, isobutane at 10.94f, and propane at -43.6, so good separation during distillation should be easily achieved. Of course distillation can’t produce 100% pure gas, but should be able to get darn close.
Show me a pic of you heating your cls up to 283f
You don’t, distill out the lighter aromatic hydrocarbons and manually clean the remainder after recovery
Edit: didn’t realize who the comment was directed too, my bad if you just tryna figure out how processors do so
Estimates of non-butane gas are upwards of 20%. Distill it that much. …
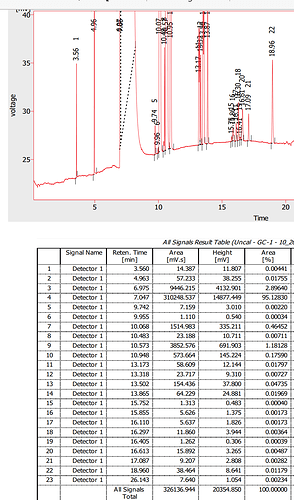
I agree. It’s nasty stuff. We ran it once through our GC for shits and giggles. Quick and dirty analysis, didn’t even want to spend more time on it.
Oh wow, that’s a lot of peaks! All kinds of stuff in there. Definitely worth it to start with a clean off solvent as possible, especially after looking at that chromatogram.
@anon16547145, I wouldn’t want any of those nasties in my extracts, and agree I wouldn’t want any in my solvent either. You don’t have to reach the high boiling points. @og_extracts explained it really well. You distill the solvent at low temp, and leave the gunk behind.
I also agree that it’s wise to start with something better than canned butane, but I don’t think there’s anything in the market clean enough to run without distilling first.
He is saying due to the higher (than propane, butane or isobutane) boiling points of the contaminants, they won’t distill over with your gas.
So what you’re saying is, There’s still a chance!
Sooo if I’m distilling canned butane ( from a great pant in England ) am I actually distilling out all of the garbage or is it likely there’s a bunch of crap left? There’s a source in my area for less than 10 pm of contaminates but they won’t sell to unlicensed extractors ( fucking Boy Scouts )
Huh? They ship that shit in the mail homie. If you’re getting mail, you can get a solvent tank shipped to you. So unless you’re living in Alaska (which I personally experienced) or Hawaii you shouldn’t have a problem getting some solvent