Agreed
Can I bring to you some of the precipitate and structures that you want to put under microscope? I can make a day trip next weekend and we can have it possibly ready for the 710 round table?
That’s what scientific rigor is all about! Please feel free to probe for holes or kick the tires as it were!
@Sidco_Cat @Dred_pirate …the common 10 Angstrom pore sized zeolite is Type 13X. The other type of zeolite commonly used for molecular sieves is Type A. This causes some confusion, since 3A, 4A & 5A are the types with 3, 4 & 5 Angstrom pores, respectively.
However, saying “10A” or “10a” for Type 13X is incorrect… because it is technically Type 13X with 10 Angstrom (Å) sized pores, NOT “Type 10A”.
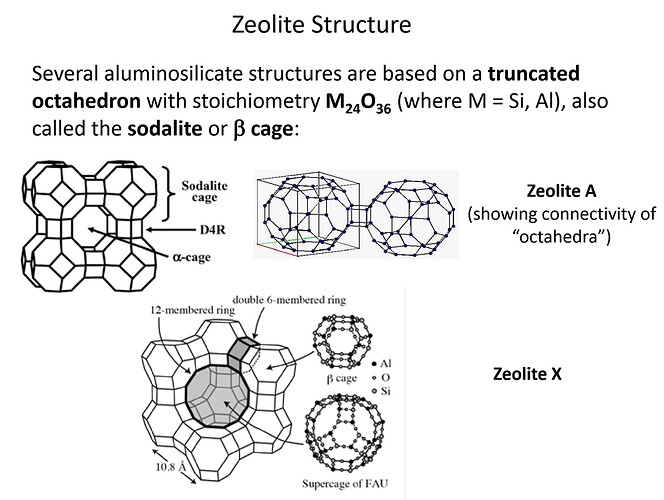
Type 13X zeolite structure is a Faujasite type (FAU)
Pore Size (Na+) = 10Å (1.0 nm)
Chemical Formula: Na2O • Al2O3 • x[SiO2] • n[H2O] …(x ≤ 2.5)
~Or~
Chemical Formula: Na86 [(AlO2)86 (SiO2)106] • n[H2O]
~Or~
General Type X Formula: M86 • [(AlO2)86 (SiO2)106] • 264[H2O] …(M = metal cation)
I do not know for sure, but I assume the 13 could be the Angstrom size of the pores in Type X zeolite without any cations… although 13X is usually used for the sodium (Na+) exchanged form, which has ≈10Å pores. Still haven’t found a definitive answer.
Here is a somewhat obscure (perhaps ESL) but helpful info-blurb I found: Molecular Sieves
I have pasted and EDITED parts of that page here for clarity:
Molecular sieve is also called zeolite. A porous aluminosilicate, there are two categories of zeolite: natural and synthetic. Its general formula is:
Mx/r [(AlO2)x(SiO2)y] · nH2O
…where n represents the number of crystal waters [water molecules of hydration; i.e. bound as part of the crystalline solid];
M represents a Metal [element] in positive ion [cation] form;
r represents the formal charge of the positive ion [e.g. + or ++];
x represents the number of metal positive ions [and alumina (aluminum dioxide, AlO2) moieties;
y represents the number of silica (silicon dioxide, SiO2) moieties].
Molecular sieve has a spatial three-dimensional structure composed of SiO4 and AlO4 tetrahedral structural units, which is characterized by uniform holes of fixed diameter, and a large surface area. After dehydration, molecular sieves have the ability to adsorb certain molecules. Molecules with a diameter smaller than the pore size/diameter can enter the pores, and molecules with a diameter larger than the pore size/diameter must remain outside the pores and may play a role in screening molecules; so-called molecular sieving.
Natural molecular sieves are crystalline zeolite minerals. The synthetic molecular sieve is made by using water glass (sodium metasilicate), sodium meta-aluminate and caustic soda (NaOH) as raw materials for synthesis and crystallization of zeolite. Then zeolite is washed, dried, (often mixed with clay to make beads) extruded or molded, and high-temperature dehydrated (aka: calcined). Molecular sieves can be divided into several types according to the connection mode and composition of the silicon/aluminum/oxygen skeleton. The common ones are as follows:
Type A: M12 • [(AlO2)12 (SiO2)12] • 27[H2O]
Type X: M86 • [(AlO2)86 (SiO2)106] • 264[H2O]
Type Y: M56 • [(AlO2)56 (SiO2)136] • 250[H2O]
Type A and Type X molecular sieves are divided into several size types.
For example, Type A is divided into
type 3A (pore size = 3 Angstroms or Å; 0.3 nanometers or nm; 300 picometers or pm),
type 4A (pore size = 4.2Å; 420pm),
type 5A (pore size = 5Å; 500pm).
All Type A zeolite has the same skeletal aluminosilicate crystal structure, but exchanging different elements for the positive metal ions (M in the formula) is what varies the pore size/diameter:
type 3A has large potassium (K+) positive ions,
type 4A has mid-sized sodium (Na+) cations,
type 5A has the smallest calcium (Ca++) ions.
Molecular sieve not only has strong adsorption, but also has good thermal stability and acid resistance. In addition, it has the advantages of cheap raw materials, simple preparation process, and easy regeneration (reusable), so molecular sieve is widely used in many industries and scientific research departments. Its main uses are as excellent adsorbent and desiccant, for the separation and purification of substances, and importantly as a catalyst and catalyst support/carrier. At present, Type A molecular sieve is mainly used for gas drying and purification, oxygen enrichment and light oil dewaxing. X-type and Y-type molecular sieves are mainly used as catalysts or catalyst carriers, and are often used in the catalytic cracking of petroleum. They have good effects and strong selectivity.
Efficient adsorption characteristics
Molecular sieve has highest affinity for formal polarity (such as that of H2O, NH3, H2S), and some affinity for momentary polarity (as in CO2, NO2, etc.). Molecular sieves of all types are especially fond of WATER, for which they maintain high adsorption capacity even under very harsh conditions such as low partial pressure (even below 133 Pa), low concentration, and/or high temperature (even above 100℃).
1. Adsorption at low partial pressure or low concentration
When the relative humidity (RH) in air is 30% or lower, the water absorption capacity of molecular sieve (max cap ≈21-26% by weight) remains almost maximum and higher than the capacities of silica gel and activated alumina at 30% RH. At very high relative humidity, the equilibrium water absorption capacity of silica gel (max cap ≈40%) is higher than that of molecular sieve, however, silica gel and activated alumina (max cap ≈5-15%) have increased adsorption capacity ONLY with increased humidity–their adsorption capacities are very small when the relative humidity is low. With the decrease of relative humidity, the superiority of molecular sieve becomes more and more obvious.
2. High temperature adsorption
Molecular sieve is the only available high temperature adsorbent. At 100℃ and 1.3% relative humidity, molecular sieve can still adsorb 15% of its weight in water. This is 10 times more than activated alumina can adsorb by weight under the same conditions, and over 20 times more than silica gel. In other words, molecular sieves can still adsorb a considerable amount of water at higher temperatures where activated alumina and silica gel greatly lose their adsorption capacities.
3. High-speed adsorption
The adsorption RATE of molecular sieve for polar molecules such as water at low partial pressure or concentration is also much higher than that of silica gel and activated alumina. At very high relative humidity, the water absorption rates of silica gel and activated alumina are similar to the water adsorption rate of molecular sieve, but with the increase of the linear velocity of adsorbate (i.e. increased flow speed of carrier gas or liquid), the water absorption rate of silica gel is less and less efficient than that of molecular sieve. The water adsorption rate of activated alumina remains about equal to that of molecular sieve in the same conditions, but alumina’s overall water capacity is quite low.
Catalytic performance of molecular sieves
Molecular sieve crystals have a uniform pore structure, and the pore size is equivalent to that of many common molecules. They have a large surface area and the surface polarity is very high. The cation that balances the negative charge of the skeleton can be ion exchanged to adjust pore size/diameter. Additionally, some metals with catalytic activity (e.g. silver, nickel, palladium, iron, etc.) can also be exchanged and introduced into the crystal as cations, then reduced to the element state. This yields extremely high (single-atom level) dispersion and equal spacing of the catalyst metal atoms on the crystal skeleton. The molecular sieve structural stability is very high. These properties not only make molecular sieve an effective catalyst carrier/support, but also help to enhance catalytic capabilities.
Properties of Type 13X
Status: yellow globular
Si-Al ratio: ≈2.6-3.0
aperture (nm): ≈0.9
bulk density (g/ml): 0.72
Residual water content (wt%): 0.56
Bead Diameter (mm): 3-5
Na2O wt%: 18.67
specific surface area (m2/g): 456
Application
Dedicated to the purification of feed gas (simultaneous removal of H2O and CO2) and hydrocarbons in large and medium-sized air separation plants.
Storage conditions
Room temperature, sealed in moisture-proof airtight containers; relative humidity less than 90%;
It is NOT to be stored exposed to open air; Avoid liquid water, strong acid and strong alkali;
Adsorption (especially of water) is exothermic. Rapid flow of humid vapor through molecular sieve can cause EXTREMELY HOT surfaces of molecular sieve beads and/or vessels containing them!
EDIT:
Activated Alumina as Desiccant.pdf (1.2 MB)
This paper discusses the adsorption modes (specifically of water in various phases) of various types of activated alumina. It is complicated, but worth a read to further grasp some fundamentals of adsorption in general (like the difference between molecular, Knudsen & Fickian adsorption/flow, as well as non-isothermal and Freundlich or BET isotherms).
…
This page has more detail on the Type A sieves and Type 13X sieve: Type A and X Zeolites
…
This is a detailed zeolite type FAU analysis: https://archive.ph/20121214221249/http://helios.princeton.edu/zeomics/cgi-bin/view_structure.pl?src=iza&id=FAU#selection-409.0-433.3
Pore characterization quantities (for zeolite Type X faujasite):
Largest Cavity Diameter (Å): 11.9
Pore Limiting Diameter (Å): 6.7
…
This page indicates Type A and Type X pores/cages: 3A, 4A, 5A, 13X... What's the Difference? - Hengye
And as if it wasn’t already confusing enough, Type 10X has 8Å pores!
…
One more thing…
The supercage is also called the alpha or ɑ Cage
Yes, @Dred_pirate , please bring ANY samples you want us to see! ![]()
I’ll reach out to directly and arrange it for this weekend.
Looks very familiar ![]()
People are still trying to figure this out??
Getting around it, no. Many of us have been able to do it for a while. Knowing what it is, no, we don’t have anything published yet.
Any other facility’s need help fixing this don’t hesitate to reach out!
Hey guys so this is my first time posting on here just made an account because of this very reason. I have been reading this from the beginning. I have to admit reading some of the posted articles made me feel less than adequate to be posting among big players in this thread. But yes just like the original post im getting this chalk on my diamonds when exposing to heat. I believe it is a cold crash that im doing. I run a X10 and have never had issues with it in anyway until recently. So can anyone confirm that this is a molslev issue and I should change my beads to 10 A beads or was the problem solved by changing solvent companies
This is just the start but yes the ovens are full of diamonds completely covered as chalk This picture is from today and this is the result from separation at 72 Fahrenheit
Things that will help people help you:
Where is your lab?
Who’s your solvent supplier?
Do you use inline filtration?
Do you distill your gas?
Try to use the little pencil to edit your post and reload that photo. Let it completely upload before you post it.
Welcome to the future @MushLove369! Give Solvent Direct a call at 833-PUREGAS, and we’ll get some of our new X-Gas in your hands to see how it can improve your process and get you back to business as usual.
Hey Guys currently having issues with my pours cold crashing almost instantly and making a hard/sticky chalk undesirable consistency we have always ran the exact same way and exact same material for years no changes I’m super confused what causes this and how I can stop this from happening as we can’t make any fse currently and only shatter here is a photo of what it looks like. We run 70/30 butane propane blend and inject -45 into our material columns through crx filter and everything has always been fine not a single issue until now . I’m up in Canada we recover our solvent via MVP through moi seive beads.
Rex in pentane
Yup I got pentane on the way. Am just curious why this is happening all of a sudden with no change whatsoever. Thanks for your reply !
The change is in your gas, not your protocol and practices. While it may be new to you, this has been an issue for a while. Check out the Medusa and fast crash threads for more detailed explanations and speculation (maybe a solution or two as well).
Get new gas from @adchem or @SolventDirect
They supposedly have a fix for this
Appreciate the reply