HHCA is different. Hexahydrocannabinol using a hydrogenation machine. theclearcompany did this year+ ago and no hate @Kingofthekush420 110% curiousness because ive had phds that knew compounds and what not but knew absolutely nothing about the extraction processes that we do which made me scratch my head. need IR Mass NMR spectroscopy
Y’all are very confused here. 10a refers to the ring position of the double bond. It is not the acid form of d10. I suppose we need a separate name for that molecule, if anyone ever produced it.
Thanks for clearing that up. I wasn’t sure where discussion about recarboxylation came from
can be done in SPD I assume aswell as the BRs back there?
Parahexyl is a well studied analogue of 10a. It’s only structural difference being the hexyl tail which should only effect potency.
d8 is double bond at 8-9 positions
d9 9-10
d10 10-10a
d10a 10a-6a
d10a on top, d10 on bottom
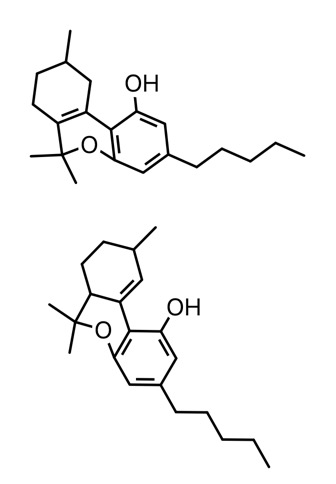
Different catalysts will yield different results. I was able to get 40% d10 using t41 in the flask twice. This is totally different. 1 catalyst in the flask will produce d10 and one will produce d10a were pretty sure, like i said were still trying to figure out what exactly is going on with part of the atom as NMR results were unclear. I was talking to an organic chemist here who says he thinks that delta 10 is stable because of the position of the bond so itll crystallize by itself unlike delta 9. Were still trying to figure this out. Weve only been running tests for about a month.
I am a natural product chemist dood send me the nmr haha.
Also stability of the compound does not correlate to packing structure of the compound. Ie because the compound is stable this does indicate whether it will be a crystal or not
@extractepic has them ive only seen them
What does it do when ingested?
Does it get you high, if not, are you doing studies to distinguish effects from placebo?
We are currently working on these type of studies and additional research. The labs at Fusion Farms have the most D10 and highest purity material of this isomer at this point, to my knowledge. And with the right partnership offering and willingness to sign an NDA, we’re open to collaboration.
Just saw this lab result on some distillate from thehempbarn.com, very high CBC and wondering if it could be in fact delta 10.
Looks like they’ve figured out a way to convert to CBC/delta 10 consistently and produce compliant full spectrum distillate.
That could be CBC, although I’ve never seen it that high in potency even post distillation. But I’ve never targeted production of that cannabinoid either. I’d want to see the crude analysis prior to distillation to accurately hypothesize if it was truly CBC.
We’ve had a couple of labs who misidentify our Delta 10 as CBC; on basic HPLC analysis it is easy to confuse because the cannabinoid retention times are extremely close. However under more thorough HPLC testing with DAD, and/or other higher power analytics, we can observe indicators that confirm it is D10 not CBC.
qNMR? Otherwise, I’m not sure how you could get a purity from your typical 13C/1H NMR
On GC, d10 and CBC are clearly different. It’s very useful to have this data, testing labs all 3 labs we use report CBC/d10 in inconsistent ways.
They’re probably going off a chromatography run with one peak. In absence of other peaks other than the target molecule one would assume it’s pure.
Theoretically if you had an compound, mixed with your target molecule, that gave no NMR signal then this approach falls flat in terms of purity.
Agreed. We have run extensive HPLC-DAD and GC-MS, as well as NMR 1H, 13C, HMBC, HSQC, NOESY and COSY. Through combining all of this data we confirmed the D10 mystery molecule, and will ideally complete an x-ray crystal structure very soon.
that is almost assuredly d10. I believe its damn near impossible to work your way back uphill thermodynamically to cbc from anything other then cbga, unless you had some way to cleave those bonds and rip that molecule open.
a lot of uninformed HPLC methods will definitely confuse them, it doesnt help that d10 and CBC uv vis is extremely similar.
You have to rely on some sort of radical. These are hard to control, whether you use photochemistry or high heat, due to the randomness of the propagation step.
