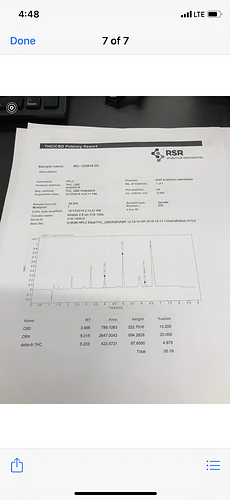
Initial distillate was 65% cbd and 3% CBC so conversion rate is not bad I will distill it to see if I can concentrated to 60% + cbn should be collected in the tails
Is the any information on the parameters for the distillation of D10 ?
How are you running your reaction? I get huge peaks of d10, if you use the proper medium cbn will elude and everything will stay,
Iv got some phoschek im going to reflux it and then distill and neutralize, dry and coa. Ill start with cbd though. See what happens
There is more than one type, in case you didn’t know. Good luck! Report back
Never mind ![]()
He lost most of his cbd
Yes it was the plan to get from the CBD to d10…
I was running same reaction on the isolate but the yield (on d10) was almost identical. And if I reflux a little longer CBCA ( not sure what my lab is calling CBCA) starting to show up and CBN starting to be created …
Is it possible what CBCA is D10a ? I will send the samples to ALEX lab to see it’s really the case.
Why neutralize after distillation and not before?
So he gets conversion while he distills too
Kingofthekush420 was 100% right ! CBD going to d9-d8 to d10 to d10a and finally to cbn… I was sampling the reaction at the 15 min intervals and test confirmed his idea.
What is this last big pick on your results ?
Any help would be greatly appreciated. I’m distilling D9 AND D 8 By accident. 57% D9 27% D8. How am I doing this? I want all D9. I filter with a layer of DE and then a layer of AC on top. I know AC in the BF will cause that, but filtering should take care of that. Any help…any…![]()
Something is making it through your DE bed
That or you’re not neutralizing an acid or something along the way
What kind of crude?
@DjTomSawyer
d8 is a result of degradation of your primary cannabinoid - that means youre looking for excess energy from somewhere. this is either coming from acidic conditions, or too much heat. “too much heat” can be too high of a temperature, or an elevated temperature for an extended period of time. surface area is also going to play a role (surface area would be increased if, for example, your crude in the flask had DE or some other media in it)
checklist
- no media in flask (including super fine carbon particles, acid activated or not)
- no acid activated AC period (trace acid salts will end up in your crude)
- distilling temperatures on your pot <220C and low vac
- time how long are you taking to distill? I have known more than one group to take too long to distill a large flask (they were obsessed with their head temp and color, just not doing a great job in general) and d8 was, actually, in a very similar ratio to what you have here. got em pushing the stuff faster and ta da, no more d8.
a big clue here is that you dont have complete or near complete conversion. if its acidic conditions causing this, its only from trace/weak acids. thats why im leaning towards too hot/too long in the flask.
Thank you so so much for your help! I’ve been reaching out to a few others in the business and they pretty much told me the same thing. I need to be completing my runs faster along with avoiding getting my mantle up too high. I will also see what kind of AC I’m using. I really appreciate the advise.
![]() my pleasure
my pleasure
BHO and ethanol crude. I wonder what could be getting through? And also, what can I do to speed up my run times?
Could your carbon be acidic somehow?
If you have any acid in there d9 will isomerize to d8