Looking to add a brine wash to remove alot of water solubles ive been extracting from hemp as of late. Lots of solids crashing out when heated that im not able to get out through winterizing filtering etc. If extracting with denatured ethanol would the correct order of events be to winterize and decarb crude, then dissolve in hexane 1:1 or 1:2 then equal parts brine solution in a sep funnel? Or is adding hexane to the solution right out of the roto with lets say 10% ethanol ok as well? When recovering solvent, is the grade recovered pure enough for reuse as a solvent or is water/ethanol content an issue if one were to reuse solvent for future washes. Thanks
Anyone ever use a 50L jacketed reactor as a large sep funnel using the stir function instead of shaking a sep fun? Is hexane the most cost effective solvent to use for this purpose?
DM me
I can only answer your 50 liter reactor question, which would be; yes. Yes, you can. I’m doing it in a stainless reactor without sight glass and that works too. So a glass reactor would both mix things better and also allow for larger batches to be ran, compared to a sep funnel. Plus you see what the fuck you’re doing as well. Added bonus there.
I would argue Pentane is a better solvent choice. It’s cheaper to recover, plus not as toxic as hexane.
It’s ideal to leave a little ethanol in the mix, it will hold on to more ethanol/water soluble impurities.
Make sure you are acidifying at least one batch of the saline with citric acid to properly dissasociate the phosphatides ![]()
So, when adding ethanol to the brine would that be discarded with the wash or kept with the pentane/heptane to be roto’d out? I didn’t know to do that. I just did my first water wash yesterday and got a lot of black stuff out with the emulsion layer, i don’t know if that’s crude or carbon.
If the initial crude is free of alcohol is the grade solvent recovered in a roto fit for re use?
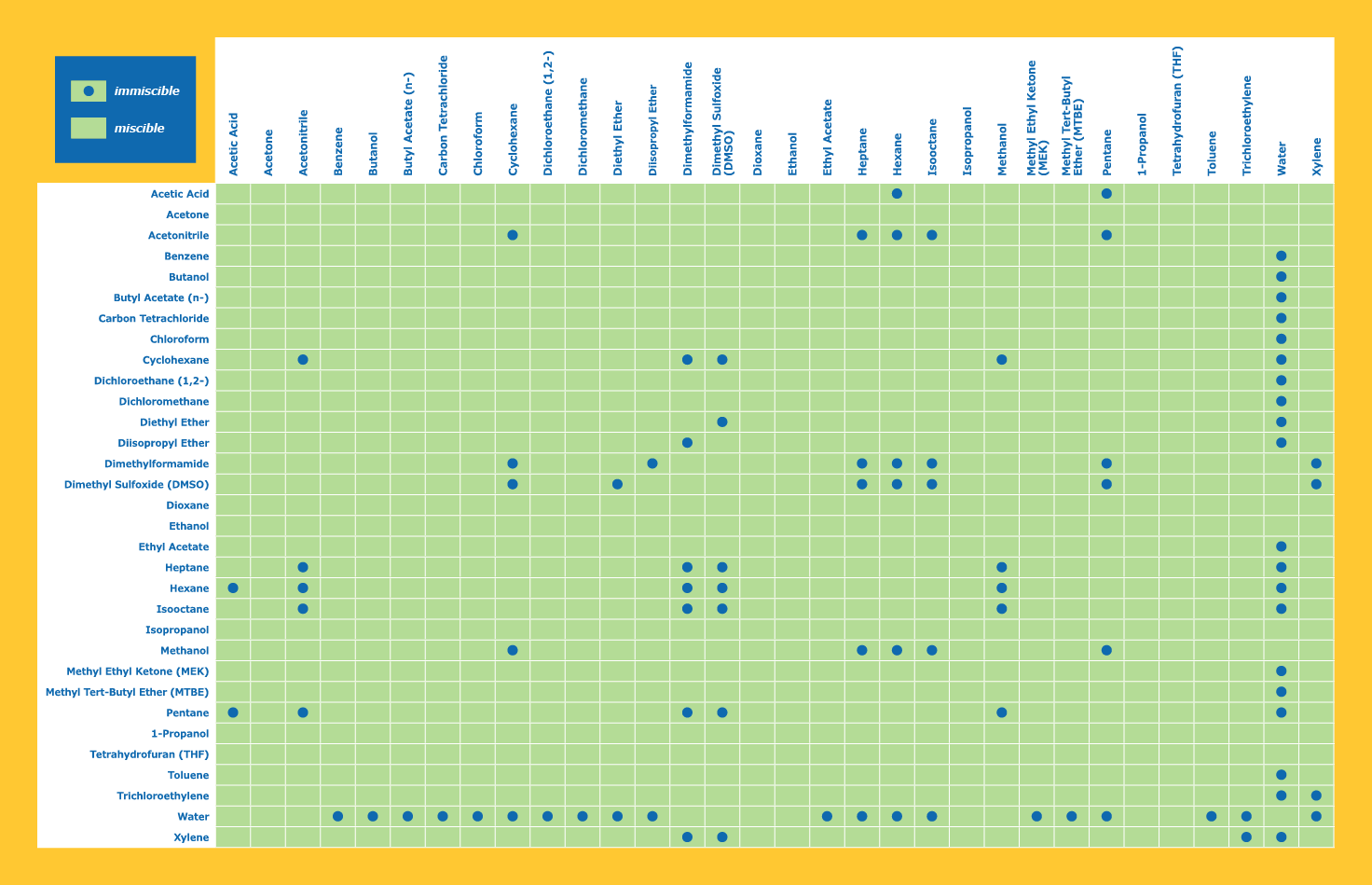
Ethanol and pent/Heptane will not mix. Water and Pent/Heptane will not mix. Water and ethanol do mix.
Either way, the hydrocarbon recovered is absolutely fit for reuse
So if going straight from a roto to a sep funnel. The trace alcohol would be miscible with the aqueous layer and only solvent of choice would remain in the oil layer?
semiPolar solvents like M/Ethanol and acetone are miscible in nonpolars like our favorite alkanes, but as soon as you add the very polar water to the solution it forces the semipolars out and into the water.
The more ethanol you leave in your solution before adding water, the higher amount of solubles will be pulled along with it.
But frankly, save yourself the trouble and chill your ethanol before extraction. it removes most of those carbohydrates among others
How much citric acid would you say is appropriate for that step?
Pentane works the best for a brine wash? Is hexane and heptane suitable as well? I currently used hexane but was told to use pentane before, pentane cost more hexane only reason why I’ve got with hexane over pentane, but if pentane is the best choice do tell and I will switch right over asap thank you
Heptane is the best choice IMO
It seperates quicker and is less toxic then the other two
Pentane and hexane both evaporate alot easier than heptane
And you can get it a some craft/art stores as the product “Bestine”.
@Kingofthekush420 your professional option is heptane I’d best solvent for brine washes. I’ll definitely keep that in consideration
Man… I remember the days of having to source small quantities of pure solvents, locally! Sunnyside naphtha (NOT VM&P), Bestine, etc. Good times!