Hello, my distillate is slightly red. How can I change it to orange or brown?
redistill it again.
Reds are typical due to sugars being pulled during extraction (especially ethanol). Degumming before distilling is recommended.
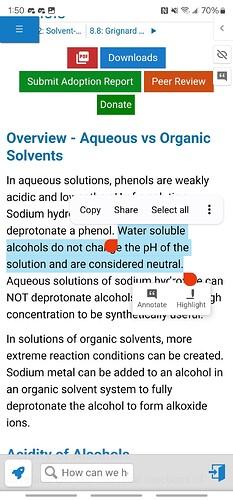
Sugars are water soluble if they were causing the reds a simple water wash would fix it
How was this decarbed? If it was done on a hot plate or something in open air your problem is oxidation
Correct. That is why a water wash will drop them out of the suspension. Just need an alkane solvent.
From my experience I’ve never had a red distillate not be red still after a water wash. Generally red is due to oxidation in my experience just like the red ring that forms on top of distillate is
By then the sugars have been “cooked”. That’s why you can run the same batch of crude via SPD and wiper and get 2 different colors.
Residency time.
Plus, water washing pretty much became a staple for me to get a neutral crude. Especially using ethanol since ethanol is slightly acidic to begin with. Neutral washes = better color and no rings.
Always degum prior to any heat application
This is not true if cannabinoids are exposed to oxygen it will oxidize even my 1000mg/g thco oxidized when left out
Yea so the color doesn’t have to do with the sugar but some other reaction with heat
Reds are not caused by sugars
If it was that simple no hydrocarbon extract could ever be red in color and ive seen plenty that are
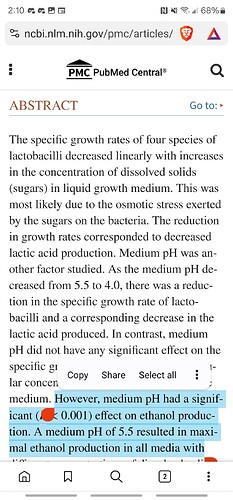
Did you skip the part where I said PH plays the part in this as well?
That’s why people’s D8 in the beginning was going red and ending up with the ring at the top. The PH was still acidic.
Sugars will cause a red twinge throughout.
When you say “your distillate”, we are talking about product you personally distilled yes?
Ph doesnt exist without water so your point doesnt apply to hydrocarbon extract
Red distillate is oxidized just like the red ring on top is from oxidation
Ph isnt what causes sugars to be extracted ethanol will extact some but the real culprit is the water that inevitably gets into your ethanol and that most people dont remove.
Ph isnt what causes old trim to extract dark oxidation is yes ph can cause it to happen faster or slower but itll still happen either way
Why do you think people pull early to get better color? Because pulling early reduces the amount of oxidation thats happening while the plant is growing
I know plenty of people who extract cold enough not to have to water wash and make good testing distillate thats yellow to orange in color
It can be either a pH or an oxidation problem. Or both.
THC in its anionic form is red/purple depending on its concentration. An aqueous wash with a solution buffered at pH 7 will reprotonate it and remove the red colour.
Some oxidation byproducts are red in colour. They form when you heat your extract up in the presence of oxygen/air. These compounds are harder to remove but can be removed with careful redistillation.
Ya’ll are both right and everything is going to be OK.
They form in the presents of oxygen period heat just speeds up the process, if it required heat distillate wouldn’t oxidize sitting in a jar
If you have a ph issue your problem isnt with the coloration of your oil and if you think remediating that is your fix well ![]()
![]() .
.
Im curious do you guys think acidic or caustic conditions make the oil red? If you are so confident that pH was the issue what was the pH of the product you remediated and what volume of a caustic or acidic reagent did you add to correct.
Ive come upon red oil many times even on same batches of crude extract but distilled in a diff manner. Ill explain my experiences when i hear yours.
Depends on the cannabinoid what the color is
Magsil + cbd + oxygen will make cbd quinone which is purple
The same thing but with thc will create thc quinone which will be yellow / orange /red depending on how much quinone is in there
Ive seen d8 that was red and you could seperate out the red through distillation and that was because you could fraction off the ptsa that was used, youd get a red fraction then after you would get gold eventually
Only seen this with d8 when ptsa was used though
Considering both are reagents but one is acidic and the other is caustic… ![]() gonna wait for some comments before i elaborate but i figured id add if people think their alcohols ph is the issue
gonna wait for some comments before i elaborate but i figured id add if people think their alcohols ph is the issue
Oh the answer was here i just overlooked. All ethanol is acidic btw(5.5 pH) So either everyones oil turns red unless they add a ph buffer/caustic reagent. Which we all know is not the case as many were making great colored oil before we even knew the details.
acidic reagents were also used in the conversion process to make water clear/D8 like activated bentonite clay(t41, activated charc) so again dont see how distilling with acidic reagents after using a acidic solvent resulted in neutral conditions.
Depending on the alcohol produced(those intended for fuel) are actually caustic themselves. These large scale mfgs arent going to alter their pH to lower their production values.
I wonder if people are still blaming medusa stones on their solvent mfg.
Because unless you have control of how the next batch is made, none of the answers above are relevant…
Is this a converted product?
Edit: yes it is… Thc after cbd isomerization
That you purchased?
Then see: Color of the distillate for viable options.
If you converted & distilled it yourself, see: D8 red
Room temperature counts as heating in my book.