Or can you suggest another method for the separation of cbd and thc?
Yeah, this will not be very usefull but gives an idea of what could possibly be done. If one was to use tertiary alcohol in basic conditions as a catalyst for d8 > d9 conversion result will be much better.
Here is the paper about CBD conversion in simulated gastric fluid. It’s interesting that contrary to their’s result we don’t see many examples of this conversion happening at such rate in vivo.
can.2015.0004.pdf (1.3 MB)
The instructions for the reaction you mention start on page 1125, for anyone who is interested.
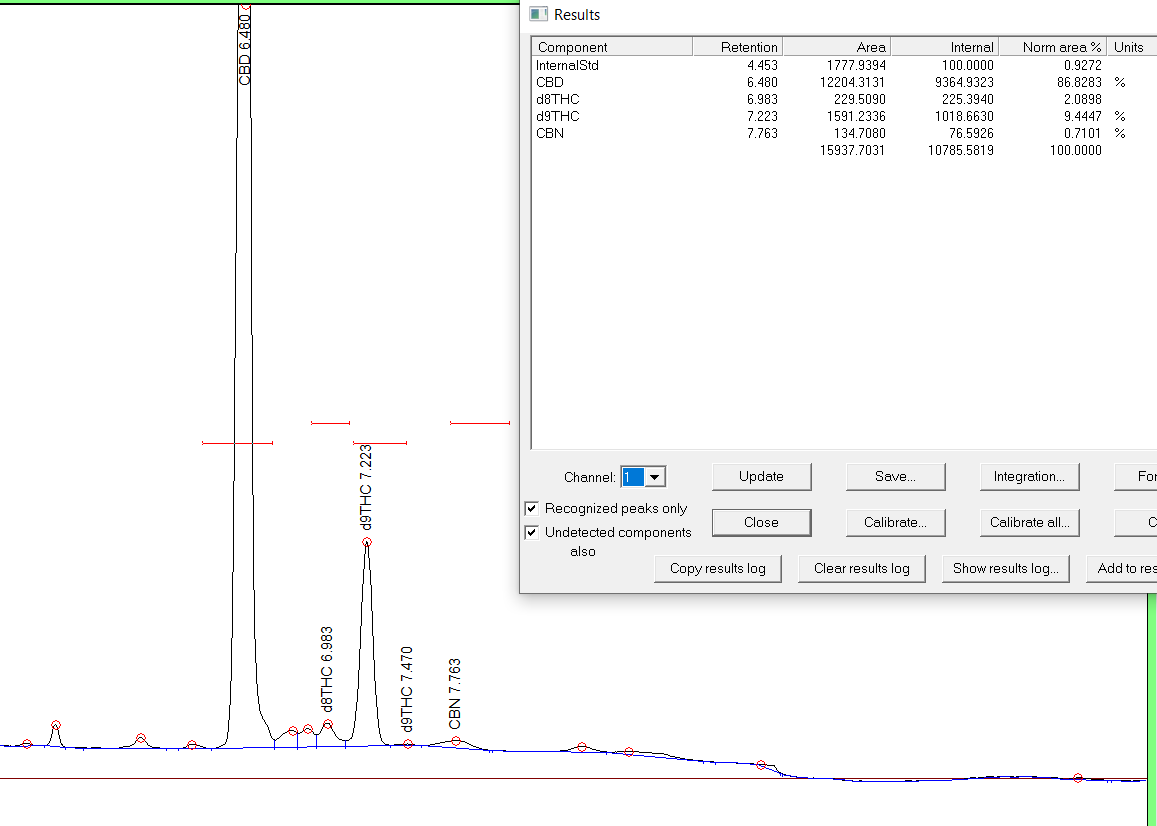
I don`t know if I’m reading the results right, but they are also yielding 19% of d8 after distillation, but then on the pictogram it says 100% conversion.
Thanks for the link, so they used the sodium lauryl sulfate as an emulsifier for the simulated gastric juice and cbd, unfortunately they don’t explain how they separated the reaction product in the end.
Imho that study is complete FUD, noone eats a tube of toothpaste with his cbd, and if children get a bit sleepy from their cbd megadoses, that is a completely normal effect. I also was a bit drowsy after vaping (not eating) half a gram of cbd.
yeah, its just as easy to trace the methodology that the industry has used over the past several years.
chromatography
In the beginning, the only companies capable of producing ‘remediated’ hemp extracts by removing most of the d9 THC were operating flash chromatography systems. Companies like interchim and shimadzu went as far as branding “420” model systems. This manufacturing process creates tons of solvent waste and requires a fair bit of knowledge about the process itself (loading capacity, how solvent systems mix, variables like flow vs temperature vs polarity vs pressure and how everything dances together) to be done efficiently. By the end of the flash chromatography era, the largest companies I had the chance to work around hadn’t even fired up their massive chromatography columns as it just didn’t make sense financially. You then see a change in COAs from CBD/CBG-heavy extract (the more polar compounds that eluted first but only gave a 40-50% efficiency per run) to CBD/CBN heavy extract because…
oxidation/conversion
It became evident as more eyes were on this high-yielding commodity (broad spectrum distillate once sold for 15k in the hemp market) that cheaper, higher capacity means of manufacturing were needed to capture the opportunity of producing the commodity. Although several different methods were developed, the whole “oven tek” thing captures this point best imo: why buy a 200k piece of pharmaceutical equipment when I could utilize a vac oven (cheap equipment already in the industry) and just slowly oxidize the D9 to CBN in the presence of heat?
Finally, as the hemp market matured to the point that it is at today, the compounds present in the “d9 separated cbd matrix” ccould be purchased in purified form, as large scale manufacturers of CBG, CBN, and then novel compounds as a whole set up shop. Want a replicable commodity with a verified supply chain for extracts that resemble broad spectrum hemp? Purchase CBD, CBG, and CBN isolates from future compounds and add them together at your preferred ratio. Just purchase your raw materials. That’s the best method of separation. The second best would be selective conversion of the compounds you want to remediate to something else. The ‘worst’ option would be large scale flash chroma and everything it entails.
Thats all cool but theoretically it would be interesting to remediate the cbd so to say, like you suggested in your previous post. It is not so expensive with chromatography, even cbd isolate is produced on a commercial scale this way, as can be witnessed on youtube.
Or you say conversion can the cbd be salted out or some shit?
Well, we played around with AlCl3 today. After 30 min, this is what we got. Not sure if we did something wrong or what. It definitely seems to favor d9 more as the d8 “peak” looked a lot more like noise than it did a proper peak. We are getting ready to throw more AlCl3 in just for shiggles. Started at 5% of CBD mass, gonna bump to 10%.
5% is enough .
After 30min you should have a complete reaction.
Greetings
this is awesome
How dry are you ?
That it s not pyrophoric doesn t mean you can run wet
Temperature of reflux ? Reaction temp ?
We did a simple hotplate reaction at 30C which is ambient here. We did not heat it at all. Just stirring. 5:1 w/w hexane:cbd. The solvent was probably not very dry. We let it run for an hour taking samples at 1,5,10,20,30,60min into the rxn.
For sample workup we washed with bicarb and the spun em on the fuge. The further into the rxn we noticed immediately that they “polymerized” as soon as we added the bicarb wash. it was so “cakey” that we had to spin them in order to actually take a sample. The first few came out fairly normal, but the last sample I ran came through absolutely bonkers looking. I’m not sure if we did not wash the sample well enough or what happened but my machine has not been acting normal. Currently trying to diagnose.
You should not use n hexane with alcl3,it isomerises to give a mixture of branched chain alkanes.
I appreciate that. It was what we had on hand to play with. Would etOH or meOH be a suitable alternative? I see that you can purchase AlCl3 in etOH solution from sigma.
Also after in the wash it’s aluminum oxide that that make it cake
That are the aluminiumoxide created as soon as you add water
With alcl3 use clorinated solvents
Really appreciate the insight. It was reminiscent of a pTSA run we did with some really waxy material. There was no catalyst in the sample but the bicarb wash for the sample polymerized what I assume to be waxes.
Would there be a better solvent than DCM?
Work dry
Dcm is fine
Lower your temp
1-Chlorobutane could be tried if DCM doesn’t go hot enough. Also maybe try zincchloride first, it sounds more preferable to have traces of zinc in the product instead of traces of aluminium.
Zinc chloride, DCM and HCL (gas). Reaction is slow as fuck but it will get the job done. As long as the starting D8 is good quality and reaction runs dry and slow one will get within 1 step to turn D8 to D9.
\
| Cost/Run Consumables | |||
|---|---|---|---|
| Material/Day | 4800 | grams | |
| C18 Silica Cost/Run | 52.5 | dollars | |
| MeOH Cost/Run | 55.46475 | dollars | |
| Acetone Cost/Run | 9.244125 | dollars | |
| Distilled Water Cost/Run | 1.485 | dollars | assumption: $.1/L |
Here are your costs with a commonly used flash chroma method, including the column material as a depreciation variable.
CBD isolate is not produced via chromatography at scale. It is produced via multiple re-x’s in a nonpolar solvent over various temperature parameters. Given the above chart, if this were the case, over 50% of the retail value of CBD isolate would be for the chromatography manufacturing step itself, under the assumption the system is 100% efficient with 100% yield, which would skew the solvent costs of the inherent chromatographic method parabolically upward.
In theory, you could actually derive the method herein via the depicted solvent cost shown above, or you could cough cough hire someone that’s done it and make your own youtube video to show off the process.
CBD could be salted out, sure. I would isolate first via the mfg method above. The resulting salt would be water soluble to boot; huge ROI increase there…
Any reason for AlCl3 over ZnCl2? trigonal vs linear geometry? more lone pairs? Only got to work with ZnCl2 with/without sieves in protic and aprotic dried solvents. Wasn’t worth scaling from the methods I tried. Interestingly, was able to achieve a 12:1 D9:D8 ratio via pTSA with about 30% CBD remaining (60:30:5) with exo as the main ‘byproduct’, but was told it wasn’t good enough and moved on.