So, ignoring DEAs definition of THCs, does that make all of the so called hot d8 legal?
No, not at all.
I think some processes can turn D9 to 4,8-iso. I know it sounds outrageous when you look at it mechanistically, but anecdotal evidence suggests otherwise.
Edit: in other words, there’s plenty of hot d8
The majority of processes have d9 as the intermediate between CBD and d8 as well, no?
According to their definition of THCs, d8 is illegal. According to their definition of isomer, the iso-THCs [d8-iso and d4(8)-iso] are not isomers of d9-THC.
The designation hot d8 implies that the d8 in the hot d8 is assumed to be legal but the hot is not.
“Legal hot d8” is an oxymoron. Actually two oxymorons.
All of them do.
I’ve been under the impression it was necessary to cycle through d9(from CBD at least), but we’ve got people claiming not to now
You could shift the double bond on cbd under inert atmosphere the same way we make d9 into d10 and it’ll make d8 cbd
You could then close the ring with an acid
You could theoretically do this to make cbnd into cbn without making thc first also
It wouldn’t be easy though
It is not only necessary, it is the only reaction pathway accessible to the substrate in its quest to turn into d8.
I strongly doubt this is feasible and for the very same reason you first have to make d9 from d8 in order to be able to make d10.
I don’t think you’re understanding me
Read the d10 paper, they take cbd and do the potassium tert butoxise reaction to it to make D8 cbd (they shift the double bond on cbd from the 9th position to the 8th without closing the ring)
You could then take this product and use an acid to close the ring to make d8
I’m really surprised you’re dealing in absolutes right right
If there’s a will there’s 3 ways
Edit:
What is the correct name for the compound I describe above? I know it’s not D8 cbd but idk wtf to call it because they don’t name it
I think what photon is saying is the mystery product hes testing for isn’t an intermediate product, it sounds like he’s trying to change the d9 into other things under harsh conditions
I just wanna know if it follows the normal CBD > D9 > D8 route
I strongly doubted, not absolutely discounted.
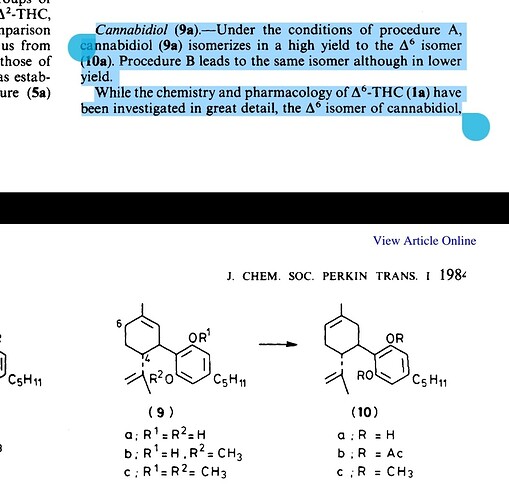
I stand corrected; The Srebnik paper do report a 60% yield of d6-CBD from CBD. I still think that is on the low side. Let’s call it barely feasible, at least I will.
And as to the naming the d6 is based on an old numbering. With the newer numbering system it would be d3-CBD.
If he’s claiming it doesn’t make d9 as an intermediate then he can’t be
Maybe you can get everything to d3 cbd using the d10 sop so when you close the ring it all makes d8
Maybe they found a better catalyst
What would chloranil do to cbd?
Think it might be able to just dehydrogenate without closing the bottom ring so we can make cbnd?
Absolutely nothing for the same reason it doesn’t touch d8.
The third ring must be in place for the 10a hydrogen to be doubly allylic and benzylic. In CBD, the average geometry of that very same hydrogen relative to the resorcinol ring is such that you cannot designate it as benzylic.
Holy shit I think I finally got it!
![]()
Sorry I’m slow sometimes
Or maybe you just facilitate formation of the iso-THC structures …
As in d8 is illegal …
![]()