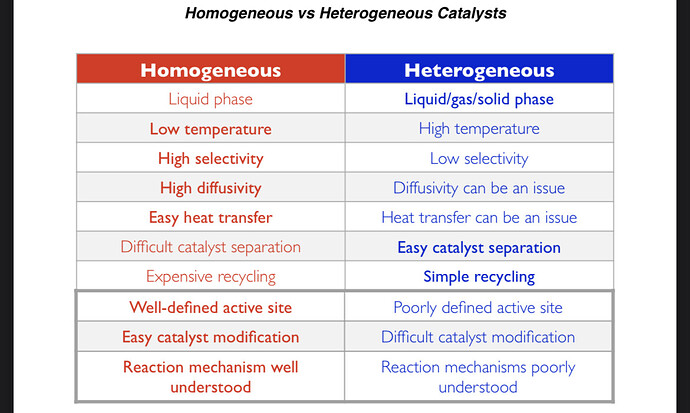
For whatever difference it makes @tweedledew stated Lewis acid as opposed to a Brønsted-Lowry acid (protic acid) which is the class TsOH belongs to.
Using too much catalyst has other unintended effects such as difficulty controlling the exotherm. TsOH comes as the mono hydrate so more TsOH means more water in the reaction, oftentimes changing things up from what can be sketched on the back of an envelope.
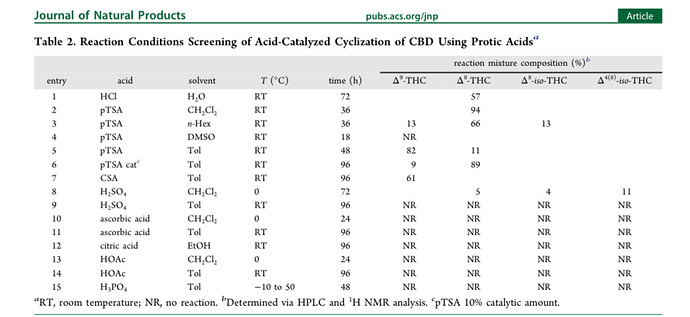
Although straightforward on paper along the lines of: supply enough thermal energy and enough catalyst to provide acceptable kinetics, there will definitely be issues at larger scales. You want to avoid exceeding the point at which the d9 turns into d8. It seems pretty clear now that this is what operators want to accomplish.
The major issue I see is adequate temperature control and exactly how and in what order reagents are mixed. You cannot simply throw in all the catalyst at once and just hope the exotherm won’t be uncontrollable.
Temperature control is not only just getting the biggest Huber money can buy. Adequate mixing is just as important and setting the stirrer to maximum speed isn’t the obvious answer.
As to mode of addition, you can either add A to B or B to A. You can add it all at once or at a defined rate. You cannot have the reaction too mixture too cold. The reaction needs to occur while addition is done otherwise you’ll end up in a situation which is quite similar to adding it all at once and once the reaction takes off, “locally, microscopically”, it will still get too hot and d8 will result.
There’s a third way of doing the “addition”, and that is to add both components simultaneously, again at a defined rate.
At the core of it, @TheGratefulPhil is right, it is about kinetics; promoting cyclization while suppressing d9 to d8 isomerization.
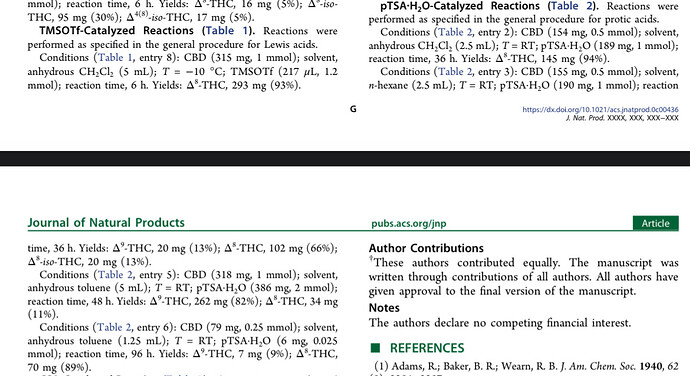
Operators want to do this with 50 kg CBD in 150 liter of solvent, not with 5 mg in a 5 mm NMR tube with 0.5 mL of solvent. Everyone can see the scaling factor for themselves and it immediately becomes apparent that it’s not surprising that the Italian paper doesn’t apply to the 10,000,000 times bigger scale.
Always wear your critical thinking cap when seeing something that you think may work. At scale, cost of reagent and reward vs. risk is obvious.
I could go on and on and on about aspects of pilot plant scale and beyond, suffice to say that without understanding the underlying chemistry, synthetic and physical organic alike, it don’t matter how well the engineering aspects are mastered. Chemistry is powerful, you are breaking and making bonds, unleashing exothermic behavior.
As with most everything science and chemistry, I highly recommend an Occam’s Razor kind of approach. Strive for economy, green chemistry, and above all, don’t flush your aqueous layer down the toilet.