Ok got it
Have you seen the nafion membrane syringe on this site ?
It s a Tsjech product and works fairly well
For D8 and a bit D9
Something like that is your best bet
Or H+. Ionic resins are a great method with litttle additional work but please read about their composition and the solvent of your choice
Im trying different hours of ozone reactions on homogenized CBD flowers and should have 3rd party tests ready in a week
all you’re making is oxidized crap which will not contain relevant amounts of THC. Your ozone generator also pumps out nitrogen oxides which react with whatever input materials there are, which would well explain the piss smell.
Feeding this shit to anyone as THC is more criminal than selling weed in a non-legal country.
This is what happens when idiots attempt stuff they don’t even remotely understand.
Million dollar question: Oxygen is a strong oxidizer and is known to destroy THC, Ozone is an even stronger oxidizer and does what exactly to THC?
it seams like you are right about the O3.
Here is a older thread about the O3 and CBD
b… but it’s in a patent!! it must be true!!! @Cheebachiefextracts @Roguelab it’s disgusting how you guys like to perpetuate shit which you obviously haven’t the slightest clue about. you are intentionally misleading less knowledgeable members and cause the very problems you’re claiming to take a stand against elsewhere. fucking hypocrisy, you should be ashamed of yourselves
This is a exploratory thread about different THC synthesis paths.
we still don’t know exactly what O3 do to the CBD and it is still pretty much a unexplored field.
That’s why I’m looking forward to the test results.
Yes, and thus your “synthesis” doesn’t belong here. You’re quoting from a bullshit patent and know nothing about chemistry, how do you think you’re qualified to contribute anything at all?
Correction: YOU don’t know. to an actual chemist it’s pretty obvious what happens: CBD gets chopped to bits thanks to the exceedingly powerful oxidant properties of O3.
CBD has way too many reactive groups to yield even a single-digit number of products from this reaction.
not that you could glean anything useful from those with your nonexistent relevant background.
As long as you do not consume it, it is fine.
You seams to know a lot about THC synthesis!
What is your option about the cold reaction with glacial acetic acid and 0.05 mol H2SO4 from a side reaction perspective?
Glacial acid are not a Lewis acid and it cannot form a covalent bond with an electron pair.
Dose this means that it create less side reactions compared to other relevant Lewis acids?
No ones lead anyone down anything here, this member brought his own projects to this thread. If you do note I also warned about o3 as well, I’ve heard stories of another member using it to do this & not quite the same results but I’m sure that’s likely due to inputs. Other than that, you said it wouldn’t do anything, yet there’s patents online for it? Just like the same patents all of us have read & used to applicate into actual tek for labs as well.
So please, before you go off with your bad attitude, get your suggestions in order please.
I almost thought I missed Roiplek, but nah.
Hi, I’m happy to be part of the community. I have few questions, if someone can point me in a right direction that would be great. I’ve read the paper, and my goal is to make high % of d9.
I have CSA and CH2Cl2 and stirred my isolate as the paper said at room temp. 48h
I received my lab test and surprisingly:
Result HPLC …
|THC|1.1 %|
|(CBN) Cannabinol|1.31 %|
|CBDV|0.2 %|
|delta-8-tetrahydrocannabinol (Δ8-THC)| 73%
It is before the vacuum distillation, so probably bit higher. I was expecting high percentage of d9 but, I ended up with some d8??? Where do I fucked up?
What would be the best solution / method? Using toluene and or PTSA?
On top of that I ate 100-110 mg of this distillate, and honestly I felt very uncomfortable. Never happened with shatter infused edibles.
The distillate also can not be smoked in raw form, it is too hard and if I put to the nail, the dabber immediately clogged???
This is not a problem anymore after adding around 7% terpenes.
Thank you all
Did you take care to dry glassware/solvents/isolate?
That paper has interesting info, but results should be considered with quite some circumspection…
CSA is quite equivalent to PTSA, just a bit slower and less specific to d8.
No, I didn’t.
I know, but that’s why I picked DCM & CSA combo.
In case I add more acid, and stir it just for 24 hours, it will be different?
Reaction time can be decreased to achieve more ∆9 or increased to make all ∆8.
Thank you.
How people reaching high percentage of d9?
D8 will be perfect for edibles, but I had bad experience. I still can’t figure it out why. Shall I eat it with some oil? 110mg of 75% pure d8 distillate should give a nice trip, right? I had high discomfort, so smoked some cbd distillate just to “relax” myself. I won’t give this to anyone else, if I didn’t enjoyed.
More acid will likely bring you to the same results, but a liitle bit faster.
People reacting for a limited ammount of time, ideally stopping at the point where all CBD has been converted, but not too much d8 has formed yet.
This 100%
I’m now starting to see some decent d9 numbers with short run times and stronger acids, this was a recent run someone I know did with ptsa and limited amount of time:
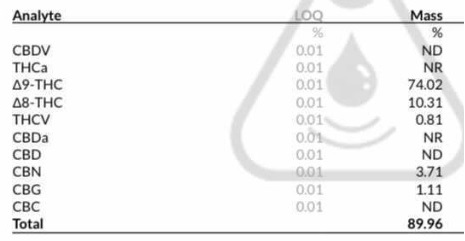
I used to think longer run times with a lewis acid like zinc bromide/chloride was best for d9 or a longer run time with a weak acid like phosphoric in heptane but now believe short and sweet is the best way.
Depends mostly on your tolerance.