2019 Jul;284:108-116. doi: 10.1016/j.plantsci.2019.04.008. Epub 2019 Apr 12.
DOI: 10.1016/j.plantsci.2019.04.008
Tables are from the supplementary data files.
This is an amazing article on its own, but offers a lot of information
about “why butane works”.
Background:
By some mysterious and undefined process, Butane extracts THCA
from Trichomes. But once you have purified THCA, most of us know
how almost impossible it is to redisolve THCA in either Butane or Pentane,
even at room temp, let alone -40C.
This is often explained with hand waving…“its the Terps”.
This article reports that there is no less than 4000 identifiable organic compounds
in Capitate Trichome heads. And below is the pdf for the list of the
MAJOR compounds found in the trichome heads of a THC varity of Cannabis.
So if the answer is “the Terps” , the answer is here:
Supplementary data_Plant_Science_after revision.pdf (267.1 KB).
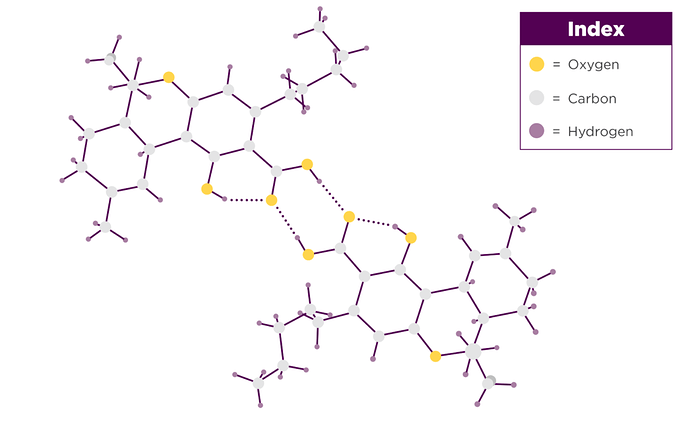
It may well be long chain amides that are known surfactants (C14 and C16).
For the brave, pop that DOI in to Sci-Hub…because the article is far
more interesting than the list. We are talking serious, analytical chemistry here.
Why the list?..some combination of chemicals in this list, either by
creating an acid enviroment in an aprotic solvent which protonates
THCA( H+), the Carboxyl of THCA, or acting as a complexing or solvating complex agent…allows THCA ( - ), ionized, to be extracted at cold temperatures by butane.
This subject has been discussed elsewhere, but needs it own
forum and requires a bit of thought.
Yes we all know butane works.
: “a few dozen hints”…It does not mean, that I know something
and am giving you hints. I am saying Nature tells us something about trichome storage area and extraction methods. Also certain scientific experiments, especially those concerning salicylic acid tell us about the chemistry of cannabinoic acids.
It is this knowledge we glean from nature which helps us understand “Butane works no theory”.
Just for clarity of word usage…R-COOH = (R-COO- H+) = protonated form = non ionized form.
apologize if the multiple ways of talking about the acid form is confusing.
On the other hand the carboxylate form = ionized form = R-COO- (minus)