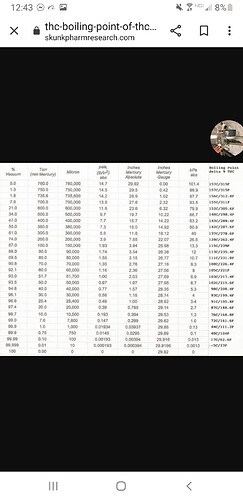
The Merck Index gives the boiling point of D9 THC at 0.02 mm/Hg (20 microns) as 200C.
not an unreasonable number. also not at “atmospheric”.
I’m asking about the screen shot from skunkpharmresearch.com that @Boshankers posted.
which lists 157C at atmosphere.
I understand you don’t have control of that site, just asking if you stood by those numbers from long ago (because why would the BP decrease from 200C to 157C when the pressure rose from 20um to 760000um).
it is @og_extracts who has the BP of THC lower than that of Azulene…
(still looking for your sources @og_extracts)
given that the all knowing one gets it wrong (Boiling point THC - Google Search), I’m not trying to bust anyone’s chops. just clarifying that THC does NOT boil before Azulene.
After looking up several sources it appears thcs boiling point is 157c , 200c, or what your claiming at 425c.
After looking up different sources my azulene BP was off. It appears to be 242.
So depending on what is thcs actual boiling point(id wager 200 over the other 2) simply cause I dab at 600ish degrees and it smokes at atmospheric pressure so I think it’s safe to say its boiling point is not near 800 degrees farenheight.
If my assumption is correct azulene would still boil off after the thc fraction.
Good points tho. I def had to double back on those sources ![]()
![]()
Hmm I wonder I got a shit ton.
Gonna try to spray it on plant for insecticide
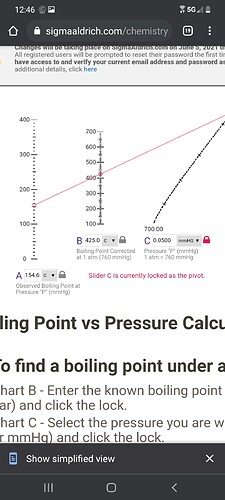
That 200C is taken at 20um…and is (reasonably) consistent with the ~425C at 760000um based on the nomograph @GroovyOctopusLabs linked upthread
I hear you on the “but dabs work…”, and am hard pressed to argue when I set my TC vape to 140C and it brings joy
So we have a group census of thc boiling point is 157c to 200c all depending on micron level correct? If so what is the groups census on the boiling point of azulene and the crap before the distillate fraction, cause I get azulene before my actual distillate fraction often and @og_extracts is saying it should come after still
That 157C BP temperature was considered correct at the time it was published and the chart was done by Skyhighler based on it.
I note that figure still abounds on line and not sure the original basis for it.
Short path distillation demonstrates that it is incorrect, but vaporization occurs below the boiling point. The boiling point is simply the point where internal vapor pressure exceeds the atmospheric pressure against it.
So in your opinion thc boiling point is 157c to 200c or 200c+ or 180c to 200c ? Ive been seeing azulene around 160c to 170c then distillate at 175c to 180c+ mainly 185c to 190c is this normal parameters
It’s fucking impossible to find any reliable data for cannabinoid boiling points so you’re excused
This is what I’ve always used as the BP of thc at atmosphere
Mechoulam says thc boils at 158 at 50 microns, that would make thcs boiling point 430 at atmosphere according to sigmas nomograph
Also, there’s a difference between evaporating and boiling
Boiling happens at the bottom of the liquid usually which is why the stir bar is so huge on an spd
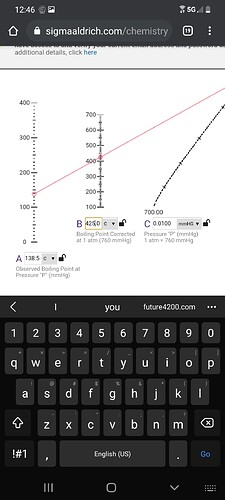
Top is 50 microns (154.6 BP on thc if it boils at 425 atmosphere)
Bottom is 10 microns (138.5 BP on thc if it boils at 425 atmosphere)
Boiling isn’t different from evaporation. It’s the same process that involves more kinetic energy and as such happens predominantly at the liquid-gas interface.
Sorry youre the only person who thinks that
For anyone who wants to do there own research watch some of the videos on the differences or even just Google it
Why would we have 2 different names for something if they’re the same?
So you’re basically taking @Cheebachiefextracts role now that he finally shut up?
i think the source of your misunderstanding is your lack of reading comprehension
the difference being
not
because boiling happens wherever there is enough kinetic energy in the liquid to exceed ambient pressure.
hence
because we’re dealing with a viscous liquid which requires stirring for good heat transfer and to prevent bumping.
Isn’t it a bit embarrassing to talk with such authority about things you don’t understand? I mean you could always watch a video or something
My understanding of boiling and evaporation is as follows:
As a liquid is heated above its freeze point, its molecules begin to move around, bouncing off the container bottom and walls, as well as its surface. It is prevented from breaking the surface and floating away by both surface tension and the weight of the atmosphere pressing against it.
The internal energy created by this molecular activity is the liquids vapor pressure.
As the liquid gets hotter, the molecular activity and velocities increase, as does internal vapor pressure, and the liquids surface tension drops.
Evaporation occurs when a molecule hits the surface with enough energy and at a direct enough angle to break surface tension, so that they float away or are carried away by air currents. It happens significantly below boiling points, especially with aromatic hydrocarbons having high internal vapor pressures naturally.
Boiling occurs when the internal vapor pressure exceeds the surface tension and the atmospheric pressure against it.
May I note that both boiling and crystal nucleation occur from points of greater energy. Typically, that is the heated surface, and small surface imperfections in that surface produce more bubbles than a polished surface. Boiling chips dramatically increase the points of bubble nucleation, and hence faster boiling rates.
My point in bringing up vapor points earlier, is to answer the question of why you can vape below the boiling point and still have effects.