Rdwc I don’t like the powders. If I could batch tank and dosatron I would but I’ve committed to the rdwc. I mean if I was doing drippers again then yes maybe think about powder. I don’t like powder in recirc systems.
Anyone have issues using athena in dirt ? Its absolutely sucked in dirt for me, except in veg in veg in dirt athena crushes it for me but when it cokes to bloom cycle it looks like its doing an amazing job but come the final finished product there’s no weight to it at all. Im.not getting the density I used to get when I would part together my nutrient line up, I am using the liquid blended line from athena with power.si and some micrcrobes from plant success suck as orca and king crab and great white.
@Onceovertwice this would work for you.
Were you using Athena Cleanse with these products?
Yes I was using the full blended line up.
Athena Cleanse is hypochlorous acid meant to kill microbes, beneficial or otherwise.
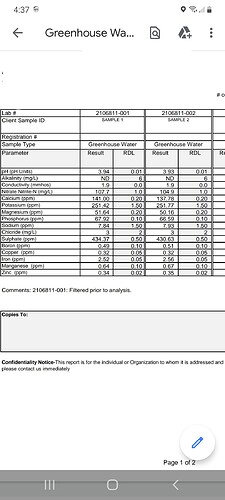
@emdub27 I just sent 2 separate samples off for testing, these are what I requested.
Greenhouse water: pH, conductance, NO3
,
P, K, Ca, Mg, B, Fe, Mn, Cu, Zn, Na, Cl, SO4
and alkalinity
Ha of course my lack of technical knowledge leads me to find out down the rode I was jjst wasting money putting microbes
Sweet.
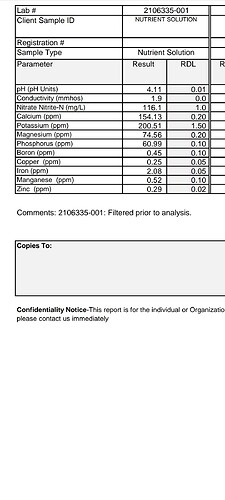
Hey guys I got back the analysis, so first thing I learned was a rookie mistake and the first sample was not taken from a big enough batch and was not accurate.
Below you see the first analysis from a sample I mixed 5 gallon of feed.
The second one is two separate samples from each a separate 55 gallon batch, I cleaned out both reservoirs before mixing the batch to ensure it would be accurate as I could get it.
I don’t have a big enough screen to compare the samples. Do you know any big differences between the two right off hand?
More potassium, less nitrogen, less magnesium. The biggest difference.
I’ll start working on a clone. I’ll probably use plant products micros for simplicity. I definitely agree with @anon56994712 and would recommend increases Mg as well as Ca.
What do you personally run for ratios compared to the Athena?
My personal stuff is greenhouse, so that changes with the weather and light intensity by season. In general if N is oversupllied to the light level, it’s fucked. Gets more interesting to keep on point when you don’t have heat and nitrate is mass flow.
It’s not wildly different than my base recipe. I can tell by the micros they fully intend to have it used at 3.0ec. Let me plug it in to excel at work tomorrow and look at it in mEq to expand further.
With the macros once they reach a minimum absolute value, antagonism and synergisms greatly change what actually gets absorbed. It’s entirely possible to get the same tissue analysis with wildly different input ratios.
So they recommended normal is 2.0 ec and heavy feed 2.7ec, all the guys I see running 3.0ec are led so I take it they run to be higher input since the transpiration rate is lower?
What I see now with my cmh or hps the feed input is working at 2.0, maybe 1.8 after week 4. That’s in coco.
Yes. Higher ec under the led for transpiration. I would increase the Ca and Mg independently.
Increasing everything is a relatively irresponsible way to do things, but the most simple.
I am going to walk through how I start cloning Athena bloom in a small series of posts. Unfortunately I can’t sit and work through it all at once because I have my standard workload. This initial post will be just getting in the computer and interpreting the obvious data from the macros. I will not be commenting on the ratios, just how to clone.
For Athena in particular right off the bat there are a couple of issues that prevent us from making an exact clone at home. 1) It is prilled as a mixture, none of us own a prilling setup and we can’t reasonably expect to make the core a homogenous mixture when the particle size of micros is so different from cal nitrate. 2) when they prill both parts they are adding additional filtering, we are using greenhouse grade salts and some insolubles are present. The worse salt for insolubles is typically SOP.
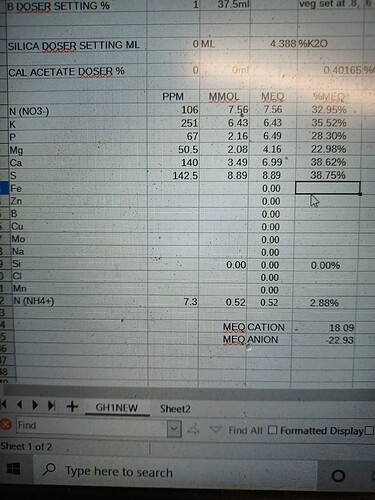
The biggest reasons I use a spreadsheet are to have the ability to look at a solution in ppm, mmol and mEq at the same time. I work with people that use hydrobuddy, so I keep my elements in the same order for easy comparison.
Most people in the cannabis industry use ppm, in academia and professional ag mmol and mEq are more common. mEq is how we calculate to a reasonable degree of certainty how to neutralize carbonates in water sources and estimate solution ec. mEq allows us to verify that a lab’s analysis actually makes sense, if we calculate 1.5ec and the lab comes back at 3ec, we know there’s a problem. It’s also how we can reconcile an all salt nutritional mix. When no nutritional acids or bases are present, the anion mEq is equal to the cation mEq.
This is what I see with the macros for Athena bloom. I assumed the NH4 based on commonly available(Yara or Haifa) greenhouse grade Cal Nitrate.
The first thing that jumps out is that we have an anion dominant mix. That means it will be acidic, that confirms what we already knew about it. The only powdered nutritional acid product I’m aware of is Pekacid. Is anyone aware of any others?
We are able to reconcile that the ec is reasonably close because anion mEq, 22.93 + cation mEq 18.09= 41.02. 41.02/2= 20.51, slide the decimal to the left by 1 number and we get a predicted 2.051 ec. All salts read differently and that’s a reasonable margin of error to the labs actual 1.93 ec.
That’s pretty much what I can easily observe. We will get in to cloning it in further posts.
So exciting thanks for sharing your thoughts and work @emdub27 ill run the copy when u post it next to jacks
There was a new acidic salt drip line cleaner that just came out, but definitely not as ubiquitous as PeKacid. I can’t remember the name for the life of me. Urea Phosphate was in the formulation perhaps?
For existing acidic line treatment for salts, there’s Pekacid, Hydrochloric acid, Phosphoric acid, Nitric acid and Sulfuric acid. For organic, there is Acetic, Citric, Oxalic, Para-acetic acids. I would just assume PeKacid is what we are dealing with.