Groundbreaking new research from the smartest researcher in cannabis extraction.
#reaching
Hahahahahahahahhahahahahahahahahhaha
Feel free to tag her if you want clarification. @murphymurri is a member here ![]()
If all you wanted to do was talk shit, this should have been created in the EC
Im asking for clarification on this hypothesis
She ain’t wrong. But she ain’t right. I’ve been to a few labs after Murphy and she does some shit wierd. But aren’t we all a little wierd?
I wonder if you could heat a Buchner with a micron filter and fullmelt and pull rosin through.
It is actually a very interesting subject matter…
But based on the non sense above…
It is going no where,
@stsndardoil… try thinking a bit about known properties of trichomes.
How about: has anyone ever measured the water content
Of freshly isolated trichomes. Before and after freeze drying.
Is everyone talking about a well defined subject?
It is a very interesting subject…and mostly because if executed correctly one can leave behind some form of white crystalline (THCa X) where x is undefined.
When I click on your links…I don’t get murphy’s hypothesis…
Can you just cut and paste.
Thx
Cannabis resin is quite literally a soup of chemical hydrocarbons. Terpenes and the essential oil constituents as a whole definitely act as a cosolvent in extraction. This is much easier to quantify using CO2 where the parameters are much more closely calculated but you can see terpenes will affect the yield of cannabinoids. They are assisting in the process, but at the same time that’s not to say that heat/pressure alone cannot isolate cannabinoids (look at mechanical separation).
Problem with this entire argument is that going back and forth about “solventless” is a complete waste of energy. To say that X ppm of butane, propane, or ethanol is more dangerous than X ppm of myrcene (carcinogen), Limonene (pulmonary toxin), or any of the many aliphatic alcohols that are present in every single cannabis essential oil (“terpenes”) is not something you can compare directly.
Hexanol and/or heptanol are present in all cannabis extracts (excluding distillate) at levels much higher than you’d see in any amount of residual ethanol on a store-bought concentrate. Before I dig up toxicity studies, why would people be happy to inhale hexanol but not ethanol? There are aliphatic alcohols in most cannabis extract that have much higher toxicity levels than ethanol. I think if people knew the chemical composition (beyond the terpenes) of cannabis essential oil then they’d probably realize that nitpicking over residual butane is a bit of a lost cause.
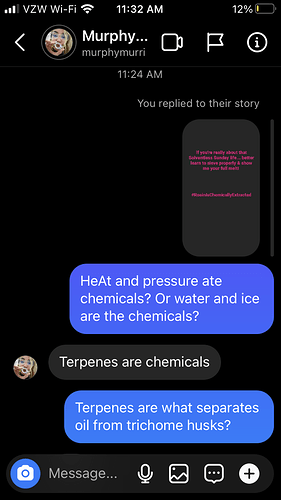
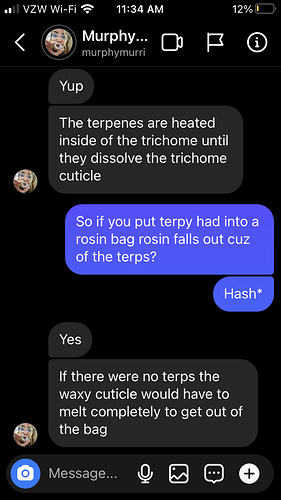
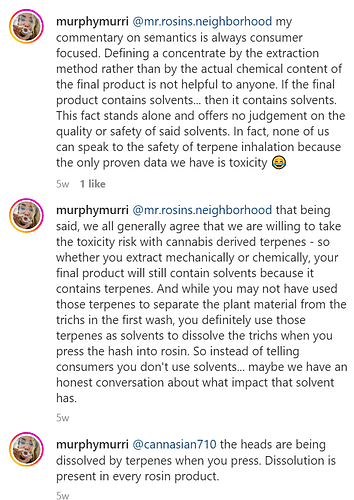
@murphymurri’s overall argument is that water hash or dry sift kief derived rosin is a process that uses solvents, so calling the process and product “solventless” is a misnomer. She suggests that the terpenes are the solvent because they dissolve the trichome cell walls under heat to release the trichome contents.
Here’s the IG link: A solvent is a thing that dissolves in aother thing…

(Me: describing = decarbing; I wish IG had an damn edit button!)
Obviously the WEEDO concept of solventless…is about as meaningful as “THCA DIAMONDS” precipitated from raw BHO.
Taking your concept “soup of chemical hydrocarbons” as a starting point (See Butane works but no theory for reference to the 4000 or more chemicals known and id by MS), when heated between two plates affording pressure release at edges,
And noting the horizontal mass transport…it can be rather interesting observation for those that are curious.
@murphymurri has an interesting argument. Maybe she’s correct about terpenes dissolving trichome cells under heat. And if so, the process involves solvents.
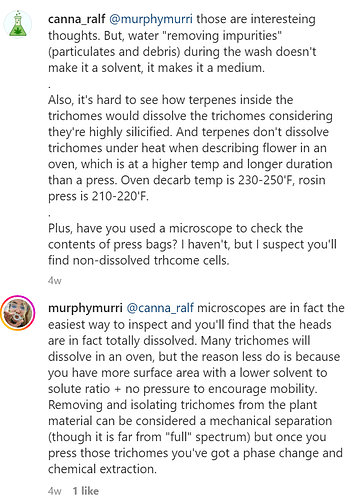
But I’m doubtful on this point because it seems to me the heat and pressure are what is melting (not dissolving) the highly silicified trichome cells releasing the contents. Case in point: when decarbing flowers in an oven, the hot terpenes don’t dissolve trichome cells.
Edit: @MassTerpenes has a good point about co-solvent activity, which may play a role during rosin pressing if the terpens are not, by themselves, dissolving the trichome cells.
Also, the argument seems two-folded:
-
If the warm terpenes dissolve the trichome cells, then the process isn’t solventless because the terpenes are the solvents.
-
The process is solventless if the warm terpenes do not dissolve the trichome cells. However, depending upon how you define terpenes, either by their nature (as solvents) or activity in the process (not as solvents), the end product would be solventless or include solvents.
All of that is true, but that’s not her argument regarding rosin. See my posts for her argument. Although she does mention the issue of terpene inhalation saftey:
I was under the impression everyone knew this and it was a generally accepted by the community.
It would be like someone in the farming community saying you can’t have a certified organic crop because waters not organic… it’s just excluded from the definition and everyone carries on like normal.
Ralph…there are a number of good papers on the anatomy of trichomes…relevant here are the apical storage structures.
I would read these papers carefully…buy a good Ziess microscope…binocular.
@Ralf “ Case in point: when decarbing flowers in an oven, the hot terpenes don’t dissolve trichome cells.” Are you trying to say heating fresh bud in an oven at 220F+ for an hour or more has no effect on the apical storage area….??? WTF?
I’m sorry…non sense.
New mantra for the 4200 BHO crew (do they really know what they are doing??):
TASTE IS TOXIC
Surprise surprise….
So if the process of rosin pressing is not solventless and the process of sieving is solventless. Can you still call the sieved hash solventless as the solvents used during the rosin press are still present in the unpressed trichomes. So the process of sieving is solventless but the product is not…
Rosin pressing is solventless.
Weed isnt by this view.
Since they naturally have terpenes and water.
Solventless was always used to describe the actions of not adding additional solvent to create a dabbable product.
We are well aware they have water and terpenes that are natural to the plant and solvents.
It’s just marketing battling the negative image of additional solvents.
Then smarty types went trying educate water hash isnt solventless and is additional solvent. Valid point, but solventless was coined originally with no Dinosaur bone solvents or natural gases in mind. Not something that can be found in the plant naturally.
Wow, @moronnabis, do you need a chill ![]() ? Or time to re-read what I wrote? You seem to be placing misdirected anger on me.
? Or time to re-read what I wrote? You seem to be placing misdirected anger on me.
@murphymurri and I had a few friendly interactions 5 weeks ago when she posted on IG. And you’ll see I never wrote she was wrong, only that I’m skeptical that warm terpenes are dissolving the trichome cells.
I posted because you asked for her position and the link. So I gave it to you. Don’t worry about thanking me or anything ![]()
![]()
I am aware of and have read many studies on trichome cellular structure. That’s why I made the point to mention they’re highly silicified. What I understand about trichomes is one of the reasons for my skepticism regarding her argument (which you call a hypothesis).
No, and I didn’t write that anywhere. I wrote that terpenes are not dissolving trichomes under heat in an oven. At least not as far as I can see. Unless when you decarb in an oven, all the trichome heads get dissolved, and you have secondary metabolite ‘juice’ on your biomass. But please, educate me if you have evidence/proof to the contrary.
Of course heat affects the trichomes, but affecting trichomes isn’t the same thing as warm terpenes inside the trichomes dissolving the trichome cells and flowing onto the plant matter.
I have to say I’m disappointed in you. You’re acting like an asshole a jerk. Your sense of superiority and sarcasm is as bad as the OPs. And this isn’t the first time your posts or posting style has come across that way to people.
I know you’re an amazing fountain of knowledge and experience. And we are lucky to have you here. However, I suggest you consider what people may infer from your tone and use of punctuation marks.
Take a cue from Graywolf, who would never write something like you just did. Yet, he, too, is an amazing fountain of knowledge and experience.