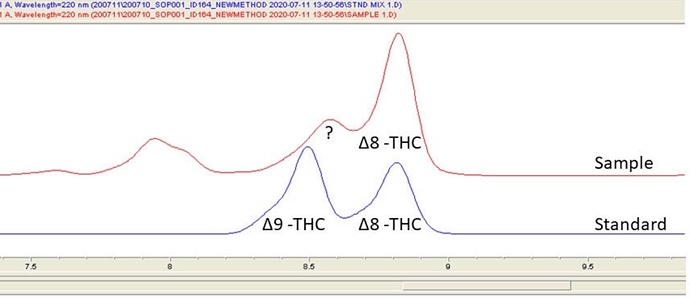
Do you know what method (acidic, alkaline, column) was used to produce the chromatogram you showed here, @Dr_Jebril? Was it HPLC or GC? What type of detector? The resolution is quite good, and we desperately need analytical labs to get back to these longer running methods for high purity isomerized samples. The method they all seem to use now elutes everything in under 7 minutes, elutes Δ9-THC right before Δ8-THC, and partially convolutes the two peaks! This is unacceptable when having D9 present (spurious result or not) means the product is unsalable under certain regulations!
We could use your help, @bigbone!
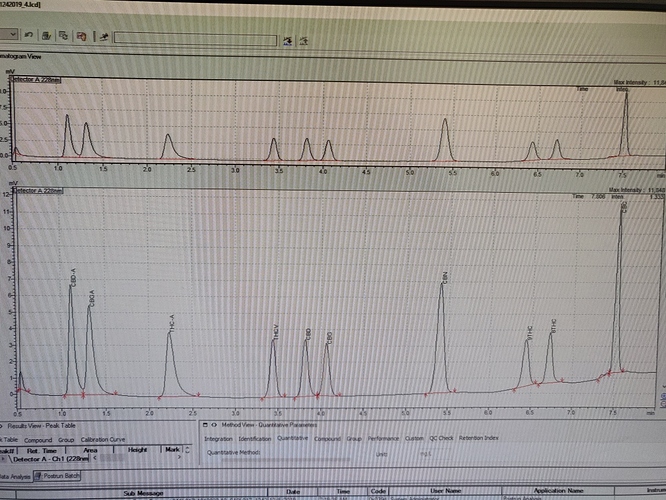
Here this is GC-FID data. It is a modified Forensics method.
The sample was dissolved into pure ethanol (~4-5 mg/ml).
The column is a 30m HP-35 (HP-5 does not separate CBC from CBD). Inlet and detector are at 250°C. It’s split injection (about 1:20 I think with 1 µl), inlet pressure is high (29 psi) and column flow varies from 3 to below 1 ml/min (as temperature increases). Carrier is He in my case. The temperature program is 80° for 2 minutes, then ramp up to 180°C (30°C/min), and further up to 280°C (10°C/min) and hold 5 minutes. For oil tincture, the ramp continues to 320°C before holding 5 minutes. The analysis takes 17 minutes for normal samples, 25 for oils. It could be made a bit faster. But I’m fine with that.
This way a large part of the terpenes are also visible, between 2 and 10 minutes.
Here’s my own hplc method, which gets baseline resolution between d8 and d9. Although they are still quite close together.
What happens to your HPLC data when you test one of these 90%+ d8 claim products?
Well, it’s been 3 years since i was running this test lab, so I don’t know. Anyone with an hplc is welcome to give it a whack.
assuming that those are his standards, a 90% sample would still generate a baseline between the two peaks. it would be 90% of that peak… why am i telling this to an analytical lab? or why are they asking actually…
also i keep hearing people complain about the infamous 7 minute hplc method that is the current standard… you absolutely generate resolution between the two samples. it looks similar to what mag chemist posted in terms of resolution between 8 and 9.
I agree, that resolution looks really good. I’m trying to find out why these other labs can’t separate them.
What’s your method looking like?
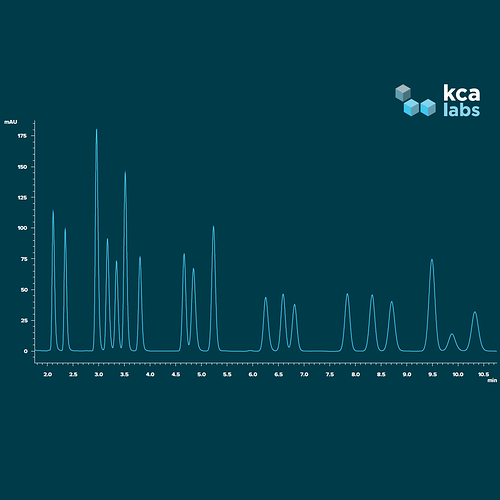
This is our standard HPLC-PDA run, 18 cannabinoids plus internal marker. We could sacrifice the number of analytes for better baseline resolution, but we’ve been throwing almost all concentrates on the mass spec to investigate the various isomers further.
Not looking bad. Which is d9 and d8?
@kcalabs This is great - displaying tremendous peak separation. To build on this, allow us to share where others may fall short, here is the issue with others -
What you see here is problematic resolution and co-elution of unknown peaks. Now let’s zoom in…
What you’re seeing is way too much variability and unknowns due to a few complex factors in testing being addressed by the hour now to fix this. This needs to be open dialogue.
They’re the 12th (d9) and 13th (d8) peaks from the left.
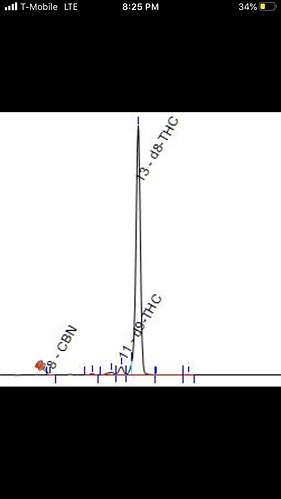
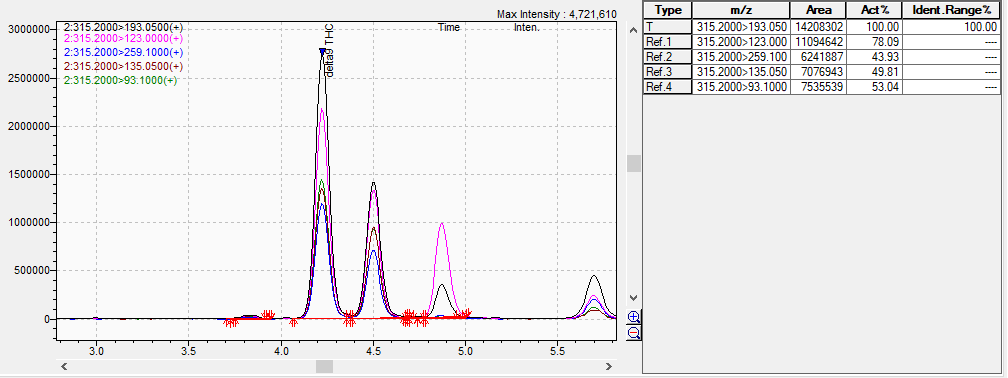
Here is the mass spec chromatogram with R=1.8, d9 on the left, d8 to the right of it.
Thanks for sharing. We’re seeing a lot of coelution around the d8 and d9 peaks in the HPLC, so we are injecting these samples on the mass spec to separate and identify the peaks.
Thanks for reaching out personally, and keep up the good work. We rely on all you guys in order to calibrate our SOP’s for ND and Compliance every day. Will share more charts with you via email ![]()
You got 18 cannabinoids in there… are there acids version in there too ?
I do only GC-FID without derivatization (no acid), and I got only 10 cannabinoids so far (as CRM). CBD, CBC, CBG, CBN, CBL, d8, d9, exo, CBDv, d9-THCv.
What else do you have ?
Yes, here is the list. A new proficiency program is about to be released to quantify 17. Who’s ready?
|18 Total Cannabinoids|
|—|—|
|Cannabichromene CBC|
|Cannabichromenic Acid CBCA|
|Cannabichromevarin CBCV|
|Cannabicyclol CBL|
|Cannabicyclolic Acid CBLA|
|Cannabidiol CBD|
|Cannabidiolic Acid CBDA|
|Cannabidivarin CBDV|
|Cannabidivarinic Acid CBDVA|
|Cannabigerol CBG|
|Cannabigerolic Acid CBGA|
|Cannabinol CBN|
|Cannabinolic Acid CBNA|
|Δ8 Tetrahydrocannibinol Δ8 THC|
|Δ9 Tetrahydrocannibinol Δ9 THC|
|Tetrahydrocannabinolic Acid THCA-A (Δ9)|
|Tetrahydrocannabivarin THCV|
The mass spec adds an additional 4 or 5 I believe.
Dude I Would watch chemist battles if that was a tv show. Crazy shit could happen!
Chemist #1 has perfected his delivery system for the Sarin gas he synthesized from roots in his back yard. Chemist #2 is unaware of just how good chemist #1 is at “getting on his nerves”!
The issue with many conversions is that if you don’t cook it down enough, too much d9- if you cook it tol the d9 is gone- then you have side products and mystery peaks
My method just cleans the mystery peaks and flushes them from the ThC fractions, slightly higher losses, but way simpler to process this way.
This is a show that @Mosaic_Co-Labs and I are writing- I’m calling it “weed heights” and the Instagram is @weedheights