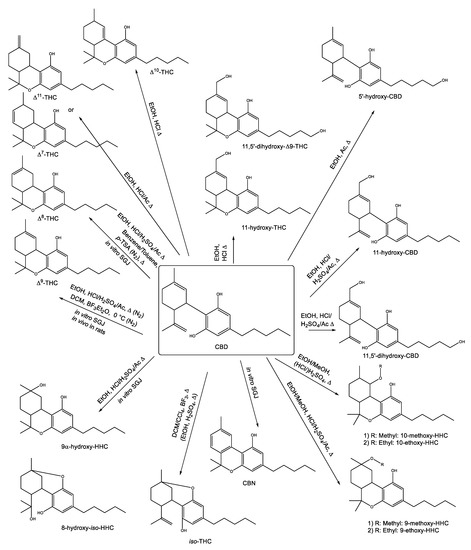
Wanted to try something: was thinking It could be possible to yield 1:1 ∆8:∆9 with a weak acid (1:5 molar equivalents acid: CBD) and 1-hour anhydrous reflux.
Hope to receive thoughts on this, or other dry reactions. Thanks!
With what acid? There are many…
Glacial acetic acid
I believe u still have to use sulfuric acid with that route never tried it for myself, i suggest u search around here plenty of usefull info with better results i suggest u read Miles-Beyond and Heisenberg run iLLnyeTheShatterGuy and others D8 SOP in real time?. I would go the phosphoric route if i were u i love the golden and sometimes waterclear after lle washes. This place is great lots of knowledge getting shared u just have to look for it (searchbar) this is a great place to start and learn good community support, only not spoon fed u have to put in the time read read read or a consultant… The are plenty around here i believe a roguelab also did them for a great price to donate to davidb funeral and kids for college,i believed…
20 mol% sounds unnecessarily high, at least if you’re considering pTSA, I think.
I’m not sure what’s super special about 50/50 d8/d9, but you can get there using an organic acid and carrying out the reaction in alcohol and optimizing the temperature for the reaction. I haven’t tried acetic acid for this purpose.
carrying this reaction out in an alcohol/protic solvent will yield hydroxylated cannabinoids. They’ll elute on an HPLC in the CBG/CBDV range and have a higher MW than THC.
It can be done somewhat successfully, although you need to look at the tertiary carbocation formed and go from there regarding your expected byproducts. You will form a few other cannabinoids in addition to d8 and d9.
THC is hydroxylated. Where would a hydroxyl group be added in that reaction, and by what mechanism?
onto C11. Golombek et al 2020. Toxics 2020, 8, 41; doi:10.3390/toxics8020041. As far as the mechanism, perhaps as a substitution after the formation of 9,11/exo and stabilization of the carbocation.
I could see these synthetic routes becoming more popular or at least scrutinized in more detail as 11-OH cannabinoids are more potent/have higher affinity to CB1 and the increase in polarity would make them easier to isolate at scale.
Check out the HU compounds here for a bit more detail on the pathways
That’s a useful review paper, thank you for sharing.
I don’t think that alcohol is what necessarily cause the hydroxylation there, I would think it would be caused by the 1)inorganic acids and 2) presence of water.
definitely agree about water, although a few of the papers referenced in that paper indicate the formation in MeOH and EtOH specifically. However, residual water in these solvent systems could be the hydroxyl donor, and that precise mechanism is poorly understood (by me) after replicating most of those reactions in a 500mL batch reactor under argon.