Ha! Indeed!!!
High entropy the solvent is in the gas phase. Low, it’s a liquid.
In my bizzy, I use the jacketed material column as an enthalpy boost coming from the frozen live resin column, feed through the media, then into the collection. Going from -60 centigrade to -5/5 centigrade, which is above my primary solvents BP
Hey man, can you tell me the necessary amount of heat needed for recovering 1lb of nbutane a minute?
I know I’ve seen the math before, but can’t seem to find it.
I’m looking to build an absolute monster of a recovery system. Currently looking at buying a 52kw, 180,000 btu propane instant hot water heater.
I saw this but am honestly having a hell of a time reading it.
At 85-90°f with 52kw of heat and more then enough cooling power through a few shotgun condensers.
Any ideas?
I gave my dad a call about it to see if he could explain it to me better. He tried… still lost.
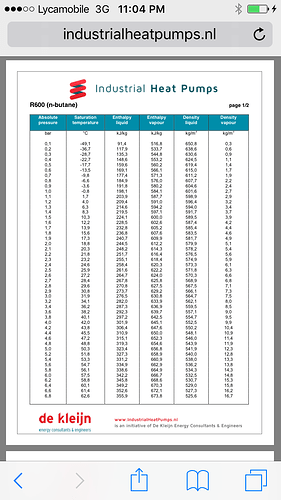
You’ll need about 3kw of heating power for each lb of butane you want to boil. In return, you’ll need that much energy to recover it, as well. Lc02 or ln2 are two of the best options for that.
Thanks man. I was planning to pump iso or acetone from my di slurry through a 6x36 tube in shell condenser. Thoughts?
Acetone probably will be hard to find a pump for. But alcohol is easy
Great point. Thank you!!!
You should definitely consider a sparkless electrical hot water heater, and completely isolated from the butane device enclosure. It doesn’t take a lot of heat to boil butane (b.p. -5°C)… that’s one reason it is a good refrigerant. The main hinderance in butane recovery is not the heating so much as it is the un-stirred pot still method of distillation. Using an explosion proof, high pressure FFE or similar high surface area evaporation and condensation would speed it up, but there are significant hazards associated with that. Just imagine if there was a leak, for example. That kind of system would have to be far away from any residents and businesses, and contained in an easily escapable fire-resistant enclosure with powerful ventilation and external fire extinguisher controls.
Please have an experienced engineer help you with any such design! Neil Ide (nyborg on Instagram) comes to mind!
I plan to use a gas one located 50’ from the source and located on the outside of the building.
My rationale tells me this would all be very low pressure at the condenser and my solvent tank would be on dry ice so we would be next to 0 pressure there. I agree with you any leak is going to be of concern, but we’re not taking more that 20-30psi tops.
If I can’t manage 20-30 psi I shouldn’t even be closed loop extracting.
I feel like I’m missing something you’re saying here.
Can you explain more clearly?
He lost me…
Glad I’m not the only one struggling with this one
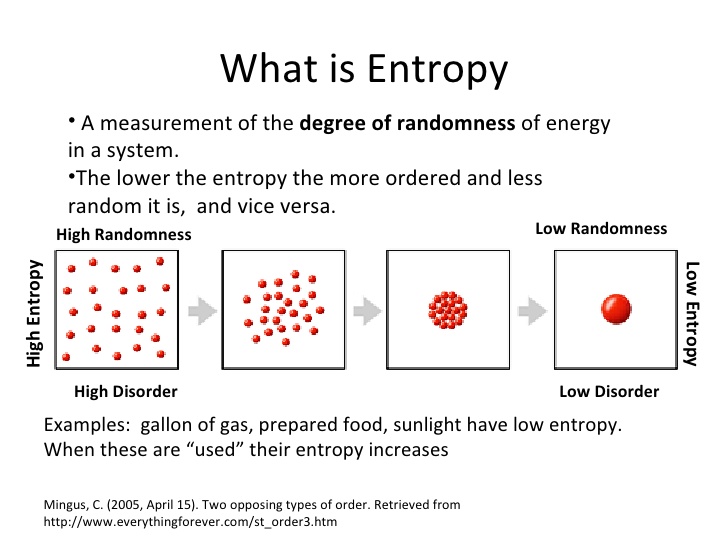
@FicklePickle, if you go to theengineeringtoolbox.com , you can see this chart for butane:
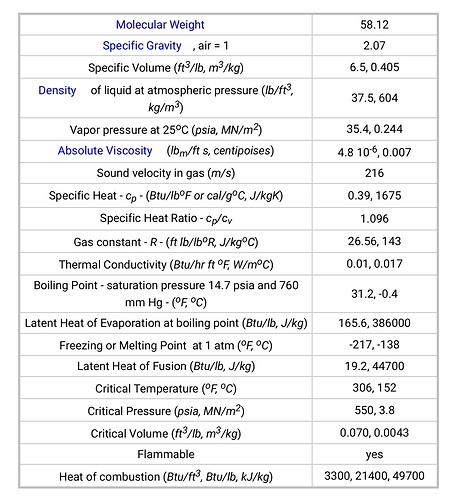
The specific heat of butane in BTU/°F*Lb. is 0.39.
This is the heat required (0.39 BTU) to raise the temperature of the unit mass (weight of 1 lb) of a given substance (butane) by a unit of temperature (1°F). This is a constant, regardless of pressure. It just so happens that you need fewer degrees to boil something with lower pressure, which is one reason to utilize vacuum.
When a substance boils, the vapor takes the heat it gained away from the liquid. So if you boil 1lb of butane, it takes 0.39 BTU away with it. Before we go further, 1 BTU/minute = 0.0175842642 kW, so 0.39 BTU/min = 0.006857863 kW… or about 7 Watts. So if you need to raise butane’s temperature by 110°F/min (from -60 to 0°C is 108°F) in 1 minute, you need 754 W/m… so 1 kW is plenty of heat, as long as it can be delivered every minute.
Thank you
I gotta get my system dialed in appropriately. But I used 100% acetone from the dollar tree 1$/6oz so I grabbed 8 of them and used three in a dry ice slurry for recovery. Dude there’s absolutely nothing like it. I’m using a ¼" recovery line and a jacketed base with ½" water lines. I have to use a lower temp on my recovery now because it’s such a nice steady zero on a flash frozen tank.
The amount of time that the recovery is “stalled” for is negligible. My solvent tank is usually so cold that it is pulling the butane from my material columns and my collection pot simultaneously. The only time I would say I encounter such a hinderance is when running nitrogen (which is almost always) and even then, the time it takes for the nitrogen to make it out the bleed valve is hardly worth complaining about. As far as simplicity, I think teaching someone the basics of thermodynamics is a hell of a lot easier and more valuable than trying to teach someone how to not run my pumps into the ground. And also just to clarify, I am not “dunking” my tanks. I run a fully automated circulating chilling system. A chiller is a better investment than a pump any day of the week. All a pump does is move the vapour. You are still required to achieve your phase changes, it’s not like you are going to be pulling a cold collection pot with your pump. You are still going to be heating your collection pot which, combined with a chilled solvent tank is as fast if not faster than having a piece of equipment (which needs to be maintained) in between. Lastly in terms of end product. I can consistently produce whichever product I want out of my passive system from crude to top quality shatter.
You don’t need to wait until your solvent tank cools down to inject your next tube. I run my system at almost 0 psi the entire time even while I am heating my collection pot, and I can inject into a hot collection pot. Your chilling capacity should be enough that you are not building a back pressure. If you are getting back pressure you have a bottle neck somewhere.
Recovery would be stalled anytime the pressure in the solvent tank is higher than the pressure in the collection pot. In other words, anytime you’re flooding a column, right?
How long does it take you to add N2 pressure to the solvent tank, flood the column and then bleed/vacuum out the pressure? It’s gotta be at least a few minutes, maybe longer depending on column size. That’s not negligible IMO, especially if you’re running a bunch of columns everyday.
Unless you turn off the heat on the collection pot and wait for the pressure to subside or you wait until recovery is almost done how do you deal with the constantly building pressure in the pot while flooding?
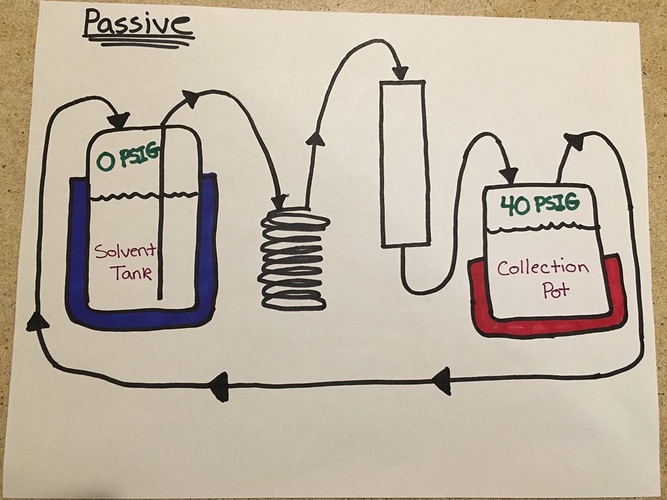
The diagram below shows my thinking. You would need to add over 40 PSIG to the solvent tank to get liquid to flow. During that time recovery cannot happen in a passive system because there is no way to work against the pressure gradient. They are inherently one-way systems IMO.
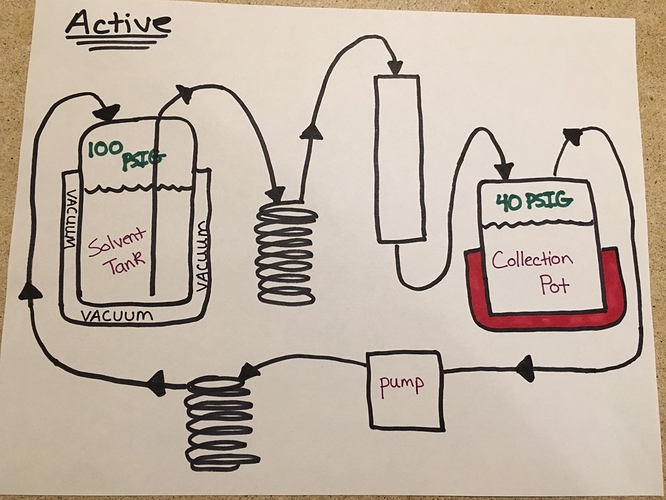
Maybe it’s simpler to just explain how it works with an active system since that’s what I’m most familiar with.
In the diagram below which is my ideal active setup, you can see the difference.
In this setup you control the amount of heat rejected by the after-pump coil by varying the amount of dry ice in the cooling bath so as to maintain 80-120 PSIG in the tank (propane) at all times regardless of collection pot pressure which itself never goes above 50 PSIG.
There is no chiller out there that can vary it’s heat rejection capacity on a dime in this manner. The amount of rejection needed to maintain 80-120 PSI in the tank is a moving target depending on other system conditions. Having the variable heat rejection capacity of a DI/IPA slurry is important to this operating method. This is even hard to do with LCO2 or LN2 because they can’t be throttled as easily as dry ice can be.
There is never any need to add pressure to the solvent tank with this method. Liquid will always flow out to the column when you open the liquid valve and recovery is constantly happening.
Warm solvent vapor is used to clear the columns and gets recovered with the rest of the solvent. Again, none of this crazy venting/vacuuming of the tank people are doing when they use N2.
You don’t need to do anything to manage this other than keep your eye on the solvent tank pressure and add dry ice as needed. The pressure doesn’t climb very fast in the tank, even with propane and my T291.
When you have a bunch of columns to run, even a little 5-10 minute routine you have to do on each one, like pressurize/vent the tank, really adds up quick that’s why I always try to make the SOP as simple as possible. Even if my recovery rate at the apex is less than a passive system, it’s more than made up for with the simpler SOP and potentially running more columns in a day.
Yes if all you want to do is set the record for fastest one-way transfer speed (or you have a continuous process), a passive setup will win. A properly sized compressor is not too far behind though.
Passive setups are inherently one-way systems as you can see in the top diagram above. Making them flow in the opposite direction is a little complicated and that’s the point I’m trying to make.
You could add multiple solvent columns like a Bizzy so you can flood from one and recover into another or have multiple solvent tanks so you flood from one and recovery into another but these solutions just add needless cost and complexity to the system IMO and add many unneeded steps to the SOP.
A properly sized compressor is not that much slower than a passive setup when you consider the goal is to run the most columns with the simplest SOP per day, not necessarily to recover at the fastest rate.
This is debatable and I would argue the opposite. Chillers have multiple pumps/compressors inside them and generally have way more components and things that can go wrong versus a basic reciprocating compressor IMO. A solid pump like a Corken, while expensive, is built like a tank and meant to last for years in very harsh conditions. Maintenance on them is honestly very minimal.
A chiller that chills a heat transfer fluid that is then pumped out to the process is also very inefficient and limits how quickly you can change the heat rejection rate at the process. I think that’s why several companies offer direct refrigeration options for LPG recovery.
IMO for medium/large scale unless you have a decently sized spit chiller system with the condenser mounted and plumbed outside the building (which takes a lot of work and money to get done) most of the “all-in-one” lab chillers that are air cooled and reject the heat out into the immediate area will not work for LPG recovery.