INTRODUCTION
It is difficult to broach this topic without getting off topic so I will keep my commentary limited, preferring to lean on direct quotes from a list of references you will find at the bottom of this post. If you are not interested in understanding what’s inside a CBD conversion, this thread will not be for you.
Some information is inconclusive and some is contentious. There are inherent scientific biases and misunderstandings as well as notorious difficulties identifying cannabinoids with similar molecular weights and retention times. This was not always as well known as it is on this forum today:
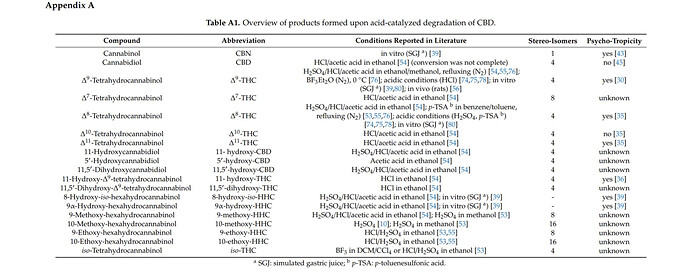
“Problems arise for small abundant compounds, such as isomers or degradation products, which could very likely exhibit similar retention times to other more abundant compounds. For example, Kiselak et al. [54] claim in their manuscript that “LC/MS analysis was able to separate all of the psychotropic cannabinoids”. However, according to the chromatograms shown in Figure 2, Figure 3, Figure 4 and Figure 5 of the respective manuscript, the LC separation shows multiple peaks and shoulder peaks under the best separation conditions and may therefore not be complete for some isobaric isomers besides Δ8- and Δ9-THC and coelutions cannot be excluded.” - Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature
This can turn cross referencing the literature into a more tedious process than it should be as assumptions must be made about additional byproducts that were occurring but not documented in research at the time. The following offers the most complete, diverse, and modern list of degradation compounds, but keep the above in mind as it references the same study:
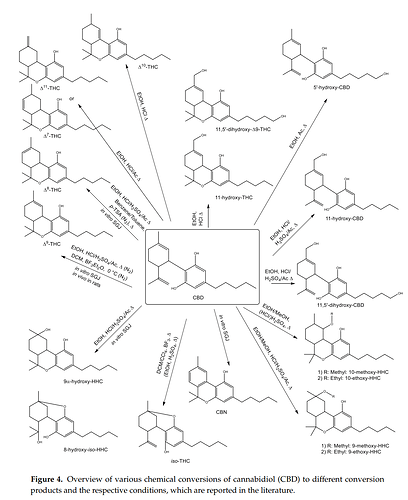
“In this study, Kiselak et al. [54] also reported on the conversion of CBD dissolved in ethanol and refluxed for 24 h in the presence of battery acid (sulfuric acid), muriatic acid (HCl) or vinegar (acetic acid). While sulfuric acid resulted in a full turnover of CBD after 4 h, the other two acids did not lead to a complete isomerization of CBD even after 24 h. Careful studies by means of ion mobility-coupled LC-MS/MS measurements enabled the detection of various formation products. Besides Δ9-THC, the products 8-hydroxy-iso-HHC, 11-hydroxy-THC, 11,5′-dihydroxy-CBD, 11,5′-dihydroxy-Δ9-THC, 11-hydroxy-CBD, 9α-hydroxy-HHC, 5′-hydroxy-CBD, Δ7-THC, Δ8-THC, Δ10-THC, Δ11-THC, 9-methoxy-THC and 10-methoxy-THC were identified (Figure 4). Peak identification was accomplished by comparison with the retention times of the reference standards and structures of unknown peaks were assigned using data from the MS/MS fragmentation and ion mobility. Yet, the only available reference substances were Δ8-THC, Δ9-THC, CBD, CBG, CBN, THCA (Figure 5a), cannabidiolic acid (CBDA, Figure 5b) and CBC. The product pattern varied depending on the reaction conditions. HCl yielded the largest number of products and exclusively led to the formation of 11,5′-dihydroxy-Δ9-THC. The reaction with sulfuric acid was the only one to produce 10-methoxy-THC and the addition of acetic acid was the only method to produce 5′-hydroxy-CBD. Interestingly, 11-hydroxy-CBD was formed in all reactions [54].”
LIST OF BYPRODUCTS FORMED DURING ACID CATALYZED CYCLIZATION OF CBD
This list is preliminary. I will update and better organize this with citations as more information is shared and gathered. Thank you to those who are willing to contribute. I hope everyone here is willing to keep discussion on topic to the identification of unknowns and to limit further speculation on their potential negative or positive effects for the time being.
I will opt for the modern dibenzopyran naming scheme but include others in some cases. It is sometimes difficult to discern certain compounds due to a different naming system used in the past.
If listed as “yes” for cannamimetic, that means at least one known configuration is psychoactive. Conveniently, in many cases it appears only the more psychoactive isomer is formed through these processes. As an example (-)-trans-∆9-THC is the naturally occuring and most psychoactive form of ∆9-THC. This is the isomer formed from CBD in acidic conditions.
9α-hydroxy-HHC / 9α-OH-HHC
MW: 332.5
Cannamimetic: Yes
Other: One of the major byproducts reported in CBD conversions. Controversially has been identified in studies with CBD and simulated gastric fluid. Occasionally seems to be mistaken for CBG. “[in mice] 8-OH-iso-HHC (10 mg/kg, i.v.) produced a significant hypothermia from 15 to 90 min after administration, although 9α-OH-HHC failed to induce such an effect at the same dose. However, both HHCs (10 mg/kg, i.v.) significantly prolonged pentobarbital-induced sleeping time by 1.8 to 8.0 times as compared with the control solution with 1% Tween 80-saline. The ED50 values (mg/kg, i.v.) of 9α-OH-HHC and 8-OH-iso-HHC for the antinociceptive effect were 14.1 and 39.4, respectively … these HHCs show Δ9-THC-like effects in mice, although their pharmacological effects were less potent than those of Δ9-THC.”
8-hydroxy-iso-HHC / 8-OH-iso-HHC
MW: 332.5
Cannamimetic: Yes
Other: One of the major byproducts reported in CBD conversions. See above re: effects
∆8-iso-THC
MW: 314.5
Cannamimetic:
Other:
Δ4(8)-iso-THC
MW: 314.5
Cannamimetic:
Other: Mechoulam mentions seeing this as a degradation product of CBC as well
Hexahydrocannabinol / Dihydrocannabinol
MW: 316.5
Cannamimetic: Yes
Other: Intermediate formed during cyclization of CBD
∆7-THC / ∆5-THC
MW: 314.5
Cannamimetic: Yes
Other: Larger amounts are more commonly synthesized from monoterpenes eg verbenol forming a racemic mixture. According to Kiselak “One significant impurity, found at 3.77 minutes, occurred in the muriatic acid method and the vinegar method. This compound is a cannabinoid peak as it fragments the exact same as both ∆9-THC, ∆9-THC. Upon further research, this peak may be either ∆11-THC or ∆7-THC. These compounds have a longer retention time than CBD, but a shorter retention period than ∆9-THC and ∆8-THC”
∆10-THC
MW: 314.5
Cannamimetic: No
Other: Typically formed by reacting ∆9-THC with a base resulting in a racemic mix of two different ∆10-THC isomers. From Kiselak: “Another significant impurity was the compound that eluted around 5.6 min. This compound was found in all the isomerization reactions and was not found in the reference LC/MS analysis. This compound is believed to be a tetrahydrocannabinol due to similar fragmentation patterns. This compound eluted later than ∆9-THC, eliminating the thought that it may be either ∆11-THC or ∆7-THC. One possible explana-tion could be that this compound is ∆10-THC, although current research is limited and currently no standard exists to confirm this theory”
∆11-THC / ∆(9,11)-THC / exo-THC
MW: 314.5
Cannamimetic: Yes
Other: One production method describes utilizing ultraviolet irradiation to isomerize ∆8-THC. As with ∆7, may appear in reactions with weaker acids. Kiselak hypothesizes this and a ∆7 enantiomer are two unidentifed cannabinoids seen in certain reactions.
∆6a,10a-THC / ∆3-THC
MW: 314.5
Cannamimetic: Yes
Other: Product of reaction with base according to literature or otherwise formed by condensation of olivetol resulting in a racemic mixture of two enantiomers. One enantiomer is shown to be the more psychoactive as with other THCs. Despite popular thought, I do not believe this is a likely candidate appearing in CBD → ∆8 conversions.
∆6a,7-THC / ∆4-THC
MW: 314.5
Cannamimetic: Yes
Other: Product of reaction with base according to literature or formed by condensation of olivetol again leading to a racemic mixture. One enantiomer is shown to be the more psychoactive as with other THCs. As with ∆6a,10a-THC I believe this is not a common byproduct seen in ∆8 conversions.
5’-hydroxy-CBD
MW: 330.5
Cannamimetic: Unknown (unlikely)
Other: Only seen in acetic acid reaction. Rarely forms otherwise if at all. Also CBD metabolite formed in vivo.
11,5′-dihydroxy-CBD
MW: 347
Cannamimetic: Unknown
Other: Seen in muriatic acid reaction.
11,5’-dihydroxy-∆9-THC
MW: 347
Cannamimetic: Unknown
Other: Forms from muriatic acid reaction and is reportedly a giveaway as to catalyst choice.
11-hydroxy-CBD
MW: 330.5
Cannamimetic: No
Other: Oxidation product formed when reaction does not take place in an inert environment. Also a metabolite of CBD formed in vivo.
11-hydroxy-THC
MW: 330.5
Cannamimetic: Yes
Other: Oxidation product formed when reaction does not take place in an inert environment. Also a metabolite of THC formed in vivo.
9-methoxy-HHC
MW: 347
Cannamimetic: Unknown
Other: Seen in sulfuric and muriatic acid reactions in methanol.
9-ethoxy-HHC
MW: ?
Cannamimetic: Unknown
Other: Similar to above but with ethanol.
10-methoxy-HHC
MW: 347
Cannamimetic: Unknown
Other: Seen in sulfuric acid reaction. Telltale sign of sulfuric acid reaction with methanol.
10-ethoxy-HHC
MW: ?
Cannamimetic: Unknown
Other: Similar to above but with ethanol.
∆6-CBD
MW: ?
Cannamimetic: Yes
Other: Forms under basic, not acidic conditions. Very little info although a study with monkeys revealed psychotropic effects. Either this has a modern name I am unaware of or it is not a common product.
SOURCES, REFERENCES, AND FURTHER READING
Forced Degradation of Cannabidiol
https://www.waters.com/webassets/cms/library/docs/720005766en.pdf
The Separation of ∆8-THC, ∆9-THC, and Their Enantiomers by UPC2 Using Trefoil Chiral Columns
https://www.waters.com/webassets/cms/library/docs/720005812en.pdf
Identification of Psychoactive Degradants of Cannabidiol in Simulated Gastric and Physiological Fluid
https://www.liebertpub.com/doi/full/10.1089/can.2015.0004
Cannabidiol: an overview of some chemical andpharmacological aspects. Part I: chemical aspects
Base-catalysed double-bond isomerizations of cannabinoids: structural and stereochemical aspects
https://pubs.rsc.org/en/content/articlehtml/2020/ob/d0ob00464b
Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization
Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature
Conversion of cannabidiol to Δ9-tetrahydrocannabinol and related cannabinoids in artificial gastric juice, and their pharmacological effects in mice
https://www.researchgate.net/publication/225788188_Conversion_of_cannabidiol_to_D9-tetrahydrocannabinol_and_related_cannabinoids_in_artificial_gastric_juice_and_their_pharmacological_effects_in_mice
Structure of Cannabidiol. XII. Isomerization to Tetrahydrocannabinols
https://pubs.acs.org/doi/abs/10.1021/ja01853a052
VASOCONSTRICTOR ACTIONS OF ∆8- AND ∆9- TETRAHYDROCANNABINOL IN THE RAT
https://sci-hub.se/https://jpet.aspetjournals.org/content/196/3/649.long
Synthesis and pharmacology of 11-nor-1-methoxy-9-hydroxyhexahydrocannabinols and 11-nor-1-deoxy-9-hydroxyhexahydrocannabinols: new selective ligands for the cannabinoid CB2 receptor
Synthetic Route Sourcing of Illicit at Home Cannabidiol (CBD)Isomerization to Psychoactive Cannabinoids Using Ionmobility-coupled-LC-MS/MS
WHO Expert Committee on Drug Dependence - Critical Review - Isomers of THC
https://www.who.int/medicines/access/controlled-substances/IsomersTHC.pdf
Hashish—VII : The isomerization of cannabidiol to tetrahydrocannabinols
Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization
https://future4200.com/uploads/short-url/4tZPIOINsOW0HA3dai0n1Ny2xkU.pdf