Federal law and policy, Hemp/CBD, Legal Issues
August 24, 2020
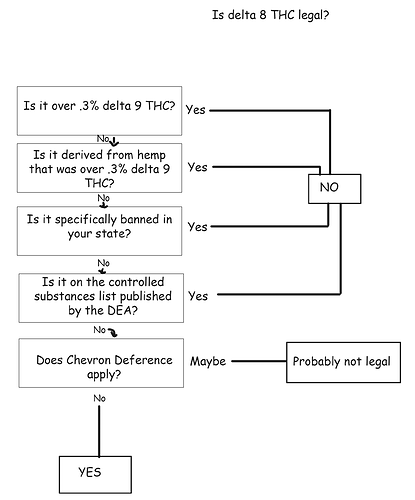
DEA Interim Final Rule: What Is “Synthetically Derived THC”?

On Friday, the Drug Enforcement Administration (“DEA”) released an Interim Final Rule (the “Rule”) that, as we discussed, threatens the hemp industry by treating partially processed hemp extract not intended for consumption (also known as “intermediary hemp”) as a Schedule I controlled substance. This is hugely problematic because intermediary hemp is an essential and necessary component of the industry.
In addition, the Rule addresses the legality of “synthetically derived tetrahydrocannabinols,” which could also impact the hemp industry. Specifically, the Rule provides that:
For tetrahydrocannabinols that are naturally occurring constituents of the plant material, Cannabis sativa L., any material that contains 0.3% or less of D9 -THC by dry weight is not controlled, unless specifically controlled elsewhere under the CSA. Conversely, for tetrahydrocannabinols that are naturally occurring constituents of Cannabis sativa L., any such material that contains greater than 0.3% of D9 -THC by dry weight remains a controlled substance in schedule I. The [2018 Farm Bill] does not impact the control status of synthetically derived tetrahydrocannabinols (for Controlled Substance Code Number 7370) because the statutory definition of “hemp” is limited to materials that are derived from the plant Cannabis sativa L. For synthetically derived tetrahydrocannabinols, the concentration of D 9 -THC is not a determining factor in whether the material is a controlled substance. All synthetically derived tetrahydrocannabinols remain schedule I controlled substances.
(Emphasis added).
Neither the Rule nor Federal law, including the federal Controlled Substances Act (the “CSA”), expressly define “synthetically derived tetrahydrocannabinols.” However, some of the DEA regulations address the issue of “synthetic THC” in the context of (1) “synthetic marijuana,” also known as “Spice” or “K2,” which is listed under Section 812(c)(d) of the CSA; and (2) the schedule I listing of “Tetrahydrocannabinol” (“THC”), under Section 812(c)(c)(17) of the CSA.
In the context of “synthetic marijuana,” which the DEA describes as a “synthetic version of THC,” “synthetic THC” refers to a mixture of plant material sprayed with synthetic psychoactive chemicals. In a 2017 Resource Guide, the DEA further explains that “[s]ynthetic cannabinoids are not organic, but are chemical compounds created in a laboratory.” (Emphasis added).
In the context of the schedule I listing of “Tetrahydrocannabinol,” the DEA revised its regulations in 2003 to specify that the term refers to both “natural” and “synthetic” THC; however, the agency’s clarification did not touch on the actual meaning of “synthetic.”
Therefore, based on the information found in the DEA regulations and publications, it appears the agency refers to the ordinary meaning of “synthetic,” which the Merriam-Webster Online Dictionary defines as a substance “relating to, or produced by chemical or biochemical synthesis.” As a result, this definition suggests that the Rule, specifically the text in bold above, may extend to hemp-derived THC cannabinoids with a Delta-9 THC concentration that does not exceed 0.3%.
This, in turn, would mean that the hottest cannabinoid currently found on the U.S. market, Delta-8 THC, would probably be treated as a schedule I controlled substance by the DEA. This is because Delta-8 THC, which is not expressed in sufficient concentrations in most hemp cultivars to make its extraction economically viable, is produced through a chemical reaction initiation by a catalyst that converts hemp-derived CBD (“Hemp CBD”). As such, Delta-8 THC would be a “synthetically derived THC” substance, in accordance with the Rule.
That’s from a top hemp lawyer… but again I’m sure you kno better