Yea I didn’t hear him mention anything about condensing butane gas with the nitrogen tho
if you just dry ice slurry ur solvent tank or use a coil the solvent will be so cold the nitro will push it into the collection making the collection that temp just long enough for you to vent off
It will be enough to move your already condensed N-Tane through your column as that’s the main function of the nitrogen push. When you freeze n-tane you get 0psi because there’s no pressure or the flow rates get stifled and need to add pressure. Most people use it to just push the stuck liquid solvent through their dewaxing column.
If you’re using a dry ice sleeved column, and you load your column with already prechilled butane that has pressure, that pressure is likely to drop in the column as soon as the pre-chilled n-tane floods the column, at which point you need to push the butane.
The nitrogen gas is not a subzero substance, it does not condense anything it’s purely just inert compressed air.
Yea the main reason I was interested in the nitrogen gas was to push my liquid butane . I was just curious if it wouldn’t also condense the little bit of butane gas that gets stuck in my collection or if I still need to vent that off into the atmosphere
I use dry ice on my tank but I still end up with pressures in my collection chamber . Didn’t no if I would still need to vent these pressures ( gasses ) off into the atmosphere when using nitrogen , or if the nitrogen pressure would be enough to condense the gas
I think you might get a condensing effect from the left over cold temps from the column but you have to burp the nitrogen before you start recovery anyways so I think you should be okay but you want to look into using a slurry of denatured alcohol and dry ice to lower the temps more first and see if that helps.
My best guess at a good answer
yes
if ur pushing dry ice cold solvent w n2 always vent the pressure off the collection the moment injection is done
the reason ppl say they don’t need to chill their collection is bc btw the dry ice cold solvent, dry ice frozen material plus a jacket filled w dry ice … just the SOLVENT moving through all this as already dry iced temp will chill ur collection
I use nitrogen at my tank but I can chill my tank to -70f and I use all the gas every time… if you don’t do this I could see moving it to the top of the material column
Everyone has to start somewhere. Better to ask a stupid question than find out the hard way. Positive vibes your way brother
By pressure do you mean the nitrogen pressure that’s left in the collection after the push is done or vent off butane pressure at the same moment that ur actively pushing the liquid butane with nitrogen ?
it will.
so now you have liquid butane, and 100PSI N2…now what?!?
you want to evaporate that butane and move it back to your storage tank.
HOW?!?
(you’ve made a little more liquid, but you’ve made the pressure problem worse. so how do you get rid of the pressure?).
Thanks ![]()
I was gonna try to stick with 70ish psi on the nitrogen
And I was just going to bleed my nitrogen off into the atmosphere before I collect my butane back into my solvent tank.
But Not sure how I will know when the nitrogen gas is completely bled off and I’m just unnecessarily bleeding off butane gasses ? I know if my collection is cold the butane will have no pressure and I can just bleed off all the nitrogen pressure and I’ll be good. But if the butane is causing pressure I’m not sure how to tell when the nitrogen is completely gone and when I’m just bleeding off butane fumes ?
Damn it , was supposed to link that last post to you
hey get your solvent colder w dry ice and prefreeze the material on dry ice then the solvent coming into ur collection via n2 push will chill ur collection enough to bleed off ALL the pressure till its at zero
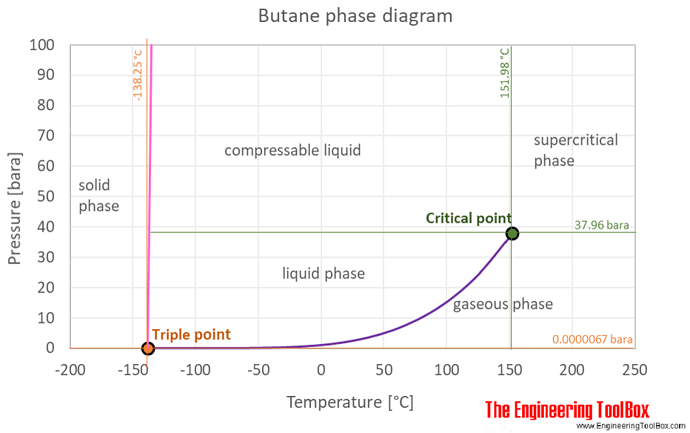
when you get the TEMP right (see phase diagram!!).
if your collection is warm enough that you have butane pressure. pressurizing it with N2 to make that last little bit liquid only works until you release the pressure. unless you remove heat (which you can do by evaporating butane…)
if there is NO N2 in your collection, why vent the pressure? said pressure is how you get the vapor to travel back to your solvent tank (which is cold, and condenses the gas)…which is the goal.
edit: …and of course you have a flammable gas detector, right?
I was saying I would vent off the nitrogen pressure from my collection chamber after the push . And that I didn’t want to vent off my butane pressure but I’m not sure how to tell when the pressure being vented switches from nitrogen pressure to butane pressure .
No I don’t have a flammable gas detector but I do my runs outside
yep.
you ALSO said
implying that BEFORE you asked about using N2, you were venting butane from your collection.
if you want to know if the gas coming out of the valve you just opened is flammable, guess what the right tool is?
does that change if you’re outside? (hand me your lighter…)
Yea but just venting the butane pressure from my collection while I’m in mid run so the liquid solvent pushes threw my crc and into my collection faster
Thanks for the tip on using a flammable gas detector to tell when the nitrogen gas is bled off ![]() . Thought you were just advising me to use one so I don’t blow my self up indoors
. Thought you were just advising me to use one so I don’t blow my self up indoors