Here are data:
I did not produce these specific samples myself.
But I’m aware of the underlying process, and I estimated they are quite representative.
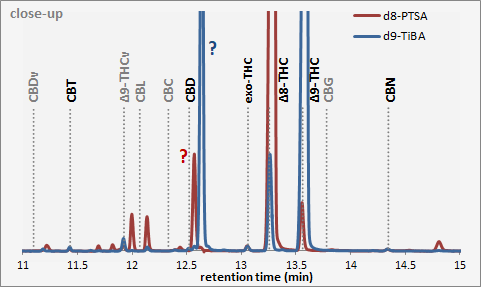
The (suspected) iso-THCs are indicated with the question marks (4.5% and 22% in the d8 and d9 sample respectively). Both samples contain a trace of the other one (and a trace of CBD as well).
If the PTSA reaction is stopped in early time, the product will actually contain more of the d9, and also more of the blue “?”… thus both are intermediary in the reaction to d8 and red “?”…
Exo-THC is 0.24% in the d8 sample, and 0.28% in the d9 sample…
In fact it does not seem to be related to the strenght of the acid.
In this paper they just say that acidic condition will push the reaction further to the last step, for both path.
PTSA produces the least of iso-THC amongst other acids like CSA, PSA, sulfonic resins… while I believe it is still a stronger acid. This may have rather to do with the morphology of the acidic catalyst.
Up to now I did not paid very close attention to the numbering in this mechanism but in fact now I realize it is mixing both monoterpenoid and dibenzobyran numberings (depending on the path).
Then if their identification of the mechanism is correct, then the blue “?” would be the delta8-iso-THC (or “delta6-iso-THC”, folowing the more conventional dibenzobyran numberings), and the red “?” would be the delta4(8)-iso-THC (or “delta6a(6)-iso-THC”). ![]()