I want to see an inorganic analysis of the pure white chalk
THCA that is produced by the solventless , resin pressing technique.
The slow heat -pressure process leaves behind a white chalk material.
I am very interested in whether this material has any inorganic ions
that need to be considered…for example any Ca++ in a 1:2 with cannabinoic acid …stoichiometric ratio. If not we can cross all the salts off our list. Or just an AES spectra of 50u trichomes…or an EDAX spectrum from a SEM of a capitate trichome head…
You need to be very careful extrapolating the species detected in electrospray from that existing in nature.
If you want large complex molecules analyzed as found you’ll probably want a MALDI-TOF where the matrix can help absorb the kinetic energy of ionization instead of creating unnatural molecules.
This dimerization is uniquely organic synthesis…
not the hydrogen bonded salicylic-dimers one finds in concentrated solutions.
synthesis of the above:
The synthesis of dimeric salicylates requires protection/deprotection steps to avoid oligomerization9. Since Δ 9-THC and its derivatives have a limited range of pH stability and rapidly undergo aromatization to the corresponding cannabinol analogues under aerobic conditions6, 7, a direct dimerization strategy mediated by the formation of an intermediate activated ester seemed worth exploring. Various coupling protocols [DCC(dicyclohexylcarbodiimide)-DMAP (4-dimetthylaminopyridine), carbonyldiimidazole, P3P (propylphosphonic anhydride), Yamaguchi coupling]10 were investigated, obtaining, however, complex reaction mixtures. On the other hand, treatment of 3a with dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP) and hydroxybenzotriazole (HOBt) afforded, in a spot-to-spot fashion, the stable hydroxybenzotriazolyl ester 4 (Fig. 2), showing that the activation and the coupling steps could not be carried out in a one-pot operational step. Accordingly, stand-alone coupling reagents (BOP and HATU) gave poorer yields, as did N -hydroxysuccinimide, a less reactive active ester precursor. Dichloromethane was the solvent of choice, presumably because precipitation of dicyclohexylurea (DCU) drove the reaction to completion, securing a rapid (30 min) conversion of 3a into 4 . The activate ester 4 could be purified by silica gel chromatography and was then cleanly converted into the highly crystalline di-depsidic dimer 5 by treatment with DMAP in dichloromethane in overall 80% yield from 4 (Fig. 2). DMAP seemingly activates the complementary “adhesive” terminations of 4 by acting as a nucleophile toward the activated ester carbonyl, and a base toward the phenolic hydroxyl.
just a note…so that no one is confused about the terminology…
above…hydrogen bonded dimers of salicylic acids…
note the “H” form necessary for the intermolecular interactions,
Duke…unfortunately you are not getting advice from Biochemists:
Look here:
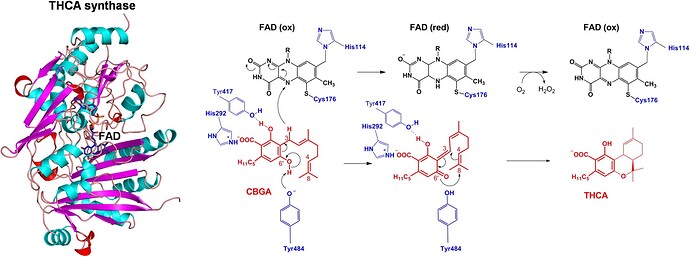
Data from XRD of CBGA bound in the active clef of the THCA synthase enzyme:
Take a CLOSE look at the CBGA molecule bound…and the THCA synthesized leaving the clef.
You need to ask…where the fuck is the H+ that neutralizes the COO-.
If you listen to those posting here…you would have to believe the scientist who did the XRD is dreaming…
original work: https://doi.org/10.1016/j.jmb.2012.06.030
YoshinariShoyama11TaroTamada1KazuoKurihara1AyakoTakeuchi2FutoshiTaura2ShigekiArai1MichaelBlaber13YukihiroShoyama21SatoshiMorimoto2RyotaKuroki1
Maybe I’m taking your question too seriously but… Water is good at donating hydrogens last I heard.
yes a totally ingenious comment…
do you understand weak acid/base buffers
and normal plant physiology?
If you actually take account of the moles of H20 in 8% moisture content dried biomass,
and compared it to the moles of THCA in 15% cannabinoid material…you would find
plenty of water …
The problem is the biomass and water constitutes a serious buffer.
Prove it to yourself by making tea from biomass…and titrate it.
But first make note of exactly what pH is of that tea…it will be 4.5 to 5.0.
Now with your knowledge of buffers…calculate the mole ratios of
THCA-COOH to THCA-COO- for a weak acid with a pKa=3.0 at pH 5.0.
now add all the water you want.
Look at the structure of the binding sites represented in this diagram (above)
has it dawned on you that the binding sites themselves only exist at a specific pH?
Can you read the diagram and tell me the pH of the XRD crystal mother liquid?
The histidine 292 amidazole is shown as protonated …but the with Histidine 114 it is not…
implying an equilibrium somewhere near pH 6…which is like 3 orders of magnitude off the
pKa value of THCA carboxylic acid…thus you notice both THCA and CBGA are denoted as
COO- ionic form. Read the methods and materials of that paper…I’m just guessing by looking at it.
Tell me what you find…this is all assuming we have a protic solvent as you suggest water…
and the entire enzyme solution is surrounded by water molecules …themselves exhibiting
multiforms depending on pH… to answer your question…yes water will do…
but in this case we do not have the appropriate concentration of Hydrogen ions to “do it”.
DO IT means protonate the THCA-COO- ionic form.
so where do they come from…or do we really need them…?
that is exactly what “Butane works no theory” means.
It would seem to me in the cleft it is bound to that histidine and as it leaves the substrate it would somehow get protonated (proton pump adjacent to maintain pH maybe?)
I also think so…but there may have to be some sort of compartmentalization.
Look at the amount of palmytamide present in the trichome…
it could also be some sort of complex…1:1 or 2:1 acid/amide…
the ir data in situ suggests the intramolecular H-bonded ring…
COOH with ortho-phenol H which is characteristic of all salicylic like molecules.
CBGA is loaded into the clef already in this form.
One clue to think about is both CBGA and THCA synthase are synthesized intracellularly
and excreted into the extracellular space. For the enzyme to be in this ionized configuration
I don’t think we can be far off pH 5.0 or so. Each enzyme with a hydration shell may serve as it own compartment. If the off-loaded cannabinoic anion is partitioned
into a secondary compartment (pH=2) which receives H+ from the pumps and then partitions
to the 3rd compartment, terp-phase as the COOH …that works for me… The H+ pumps are well known in plants. See ref. below. Also, a data base of the transcriptosome of Trichomes is available on line. If one of the mol-bio guys would do a data base search for the H+ pump proteins and see if there is a match it would be helpful indeed. There is just a number of alternatives to eliminate. If you think of crystallization of various THCA forms from various solvents as the same problem (with less variables) in reverse, you can understand my interests.
4200 in search of proton pumps? I think they are prominent in all vacuole like structures.
Proton pumps and compartmentalization may be the simplest explanation…
here is a good reference:
Q: do the buds taste acid to you?
transcriptome data: DDBJ/EMBL/GenBank under the BioProject ID PRJNA560453.
pump: P3A-ATPase (PH5)
It could very well be ion paired to another but charge neutrality dictates something.
Dick measuring contest over pka knowledge? Man good times in biochem. Young wannabe medshooler are ya?
So in vivo, the enzymes are obviously active in a water surplus environment at the various pH found in the trichomes right? You are saying the ph is not within the correct range to protonate the coo-? And your evidence of this is from the hypothetical ph of reconstituted total biomass?
What is the pH of the trichome? Why does it matter the ph of this crystallography experiment if we are trying to get at the in functionality of cbga to thca in vivo?
And try to write down a thought in a way other people could follow when you answer please. I know you have the knowledge, but your sentences are like a trump rant on paper.
thanx…your comments are helpful…
the discussion can not be “dumbed down”…and actually it is just 101 biochem/buffers…
all I can say and have said is “Butane works”.
that is really the level you need to think at.
with respect to your suggestion about water…
the answer is simple: water can only work if the pH is correct.
Yes I have measured the pH of trichomes…of course.
I’ll let you figure out the way to do it for yourself…it is a measure of curiosity.
It will bring you closer to the mystery: “butane works but no theory”
best regards…
@cyclopath we need to get the THCwtfA tested by mass spec!
Yep, and I suspect there may be larger complexes flying on our GC.
![]()
Just because it exists in your chromatogram doesn’t mean it exists in vitro (or in vivo). Especially with electrospray ionization. The forces of physics impinging upon a droplet of ions as the solvent evaporates leads to an explosion and the transmission of incredible amounts of kinetic/chemical energy that sometimes creates species that don’t naturally exist.
Would MALDI be an appropriate alternative for this?
MALDI is for huge proteins. Something like chemical ionization gives intact MH+ molecular ions.