Ok so I keep trying to read about the chalk diamonds phenomenons. @moronnabis has sent me some messages that are why over my head and I’m hoping some collaboration can shed some light on this topic. He keeps asking what form of thca I am extracting and says he forces it into thca-h+ via acid.
I have never even heard of thca-h+ besides one thread that him and a few others including photon and a few others. The question I will be searching to answer is what are the possible forms thca can be extracted in. As I am writing this I still have no idea about THCA-H+ or what other forms possible there are not hopefully with this thread we can compile the possible forms and get this information out there.
I am about to start my googling and I will post relevant links in here. If anyone else digs in or has more knowledge please feel free to share and @moronnabis thanks for your patience while I play knowledge catchup.
https://pubs.acs.org › doi › pdf
Chemical Profiling of Medical Cannabis Extracts - ACS Publications
I’m starting with these two.
@mitokid might be able to shed some light on this
From what is described …
… it sounds like there is excess of an acidic component present in the solid material. What can be gained by ensuring that all THCa is actually in its protonated form, THC-COOH, and thereby promoting crystallization, may be offset by that same excess, possibly creating unstable “diamonds” that slowly undergo decarboxylation, breaking up the crystal structure from “within”, resulting in chalky diamonds.
I know acidic conditions will speed up crystallization
It sounds like too much acid is a bad thing though
There must be a balance point
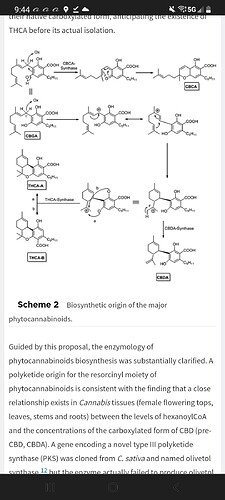
So there are 2 isomers of THCA, THCA-A(acid on the #2 carbon) and THCA-B(acid on the #4 carbon). THC-H+ is just a way of saying it is still an acid and has not lost the acidic proton or has not been deprotonated. I would rather call it THCA and assume that no charge means that it has not been deprotonated since THCA- is the proper way to write the deprotonated form of THCA. So I would say that there are 4 versions 2 with the acid on carbon #2 one protonated and one deprotonated and 2 with the acid on carbon #4. My best guess would be that the protonated forms would be much more easily extracted by butane than the ionized forms(when deprotonated they have a charge and are considered ions) and I am still unsure what would deprotonate them in a butane solution. I am going to have to do a lot more reading to catch up on the knowledge around here.
So if there is a difference in the crystal structures of THCA-A and THCA-B (there is) and all of a sudden we have a different ratio of those two isomers it could cause the diamonds, which would include all the isomers, to have a different ratio as well and could cause issues like we are seeing. However it is a very complicated idea and seems hard to imagine that the ratio could change so rapidly. There may be protonated and deprotonated molecules and ions in the crystals as well which would have a destabilizing effect on the structure. Then throw into the mix a possible contamination with pentene or propylene and who knows what would happen. The best (at least short term) solution seems to be recrystallization in Pentane or Heptane…
![]()
For naturally occurring THCa, THCa-A is for practical intents the only one you will ever encounter. I have brought this up before in other threads. I believe that Mechoulam isolated crystalline THCa-B from a recarboxylation reaction of THC with MMC (Methyl Magnesium Carbonate).
I agree that the nomenclature by OP is a little confusing. The a in THCa implies that the carboxylic acid is protonated and I interpreted THCa-H+ as indicating THCa being crystallized in the presence of an additional acidic component.
I think the best way of indicating the carboxylate form of THCa is THC-COO-.
Does it need to be thca-a and thca-b that we are seeing or could it just be a change in ratio between standard THCA and the deprotonated version?
@mitokid appreciate the info. I’ll test some diamonds from the same batch still in solvent that are not chalk vs some that have been chalk for a month now and see if I notice and significant potency different. When they are tested right after chalking the potencies on both were still basically identical.
It doesn’t take much of an impurity to cause crystal structure defects that may cause instability. The more perfect your “diamonds” appear, the less of anything else but THCa is in there.
I doubt you will see any difference in your COAs that can be attributed to what I describe.
Still not sure what was meant by THCa-H+ but if that meant that the diamonds were grown in the presence of extra acid, then it is very plausible that the component got included in the crystal growth, causing imperfect, unstable, and “chalky” diamonds.
No @moronnabis was suggesting that some of the issues people are experiencing is because we are not forcing thca into the THCA-H+ and for the life of me I couldn’t figure how that was any different from THCA but I see now that it isn’t it was just meant to hint to me I may be having issues due to deprotonated THCA. It’s worth adding none of these diamonds are being caused by strange parameters that didn’t at one point produce completely normal crystals.
Right and that is the part that is so frustrating. I believe it is a multivariable problem that can hide the fundamental issue/s. It could be a combination of Propene, Benzene, protonated or unprotonated THC-a, b all or none or some mix.
I would completely agree with this. Luckily a local lab said if they can not figure out what is going on they have connections with NMR so I’m going to drop them off samples tomorrow.
Yes NMR would be great. I have been working on a few friends of mine who are crystallographers to run XRD but so far I am not getting any response back. I have run many XRD but don’t have access to the instrument anymore and I think this being federally not legal is a huge hurdle to overcome.
So if my day of research is correct all of this would mean that thca in its deprotonated form could be forming many different salts by interacting with different positive ions available inside the mother liquid. Wouldn’t this mean that there are a bunch of different potential thca salts that are being created and sold and they all have different properties than standard thca-a? Someone stop me if I am wrong as my mind is blowing right now.
@moronnabis @DocKnott @mitokid
No, they wouldn’t co-crystallize with THCa, and personally, I think any attempt to isolate the sodium salt for example, would result in its spontaneous decarboxylation.
What’s the reason for this?
Yes, crystals often form with impurities like amethyst is quartz with iron impurities making it purple. This can happen and does happen all the time in crystals. If the impurities fit in holes in the structure it doesn’t affect the crystal structure too much but if it doesn’t the structure can be very different. If we could get XRD on a medusa stone we could know for sure but I am still having trouble finding someone who can run them. I may have someone at UCDavis we will see.
It is true that impurities usually make the structure less stable. Believe it or not sometimes impurities can make it more stable but in this case if it is an impurity it seems to be less stable which is why it degrades and chalks.
If I am able to get someone to run XRD at UCDavis is there anyone with chalked medusa stones that can bring them there or somewhere near there?