Unheated? What temperature ranges?
Even in extraction I used heat.
Unheated? What temperature ranges?
Even in extraction I used heat.
Hahaha glad you found this to be FUCKING WIERD too
Maybe you can dissolve an extract in heptane and add that mixture as well as some water to a pressure vessel and pressurize with CO2 gas?
Agitate by strapping it into a paint shaker?
What i am trying to understand is what flavors it s creation
Agitation ?
Pressure?
Singlet oxigen ?
mind you all these isomerization isseu s happend in a bucket with dry ice thats it
So you think these morons who I know have this material on hand are using carbonic acids aka using this relatively complex methods im reading about here? I’m thinking it’s a bit more layman derived because a lot of these guys with this type of material I know are idiots
It’s not complex, carbonic acid occurs in aqueous phase if you’re using wet solvent and dry ice. There is not need to produce pure carbonic acid (in fact it’s pretty much impossible).
I cant seem to find much information that I can make sense of because I keep getting articles that deal with geology.
No Defenatly not
They are using a catalyst wich can be any acid with some heat
That trick isn t a trick at. All
I would chose a weak acid like maleic acid
Pressure water and CO2 Make for carbonic acid. More pressure makes for more acid. What exactly is the issue with doing these intentional isomerizations under SCO2? It generally makes for high propagation rates which is why they use SCO2 to speed reactions in a bunch of pharma/chem applications. Making the stuff is easy, keeping it after you drop the pressure is not.
How are you recovering your solvent at -78°C?
You can use dry ice as a source for carbonic acid when there is H2O present.
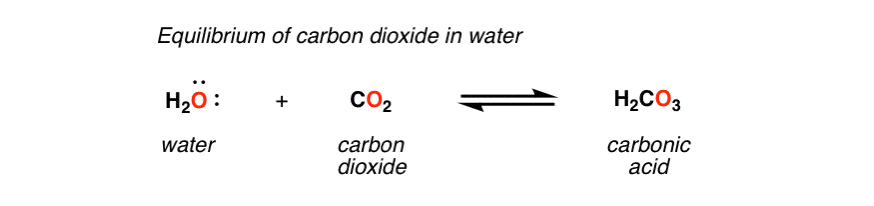
To keep the carbonic acid in solution, pressure plays an important role.
Interesting that it caused isomerization without any heat activation.
I’ve used dry ice as a means to do a base neutralization. Add dry ice to your basic solution and the carbonic acid will react to form water and stable carbonate salts.
Coa s are from spend biomass ![]()
the man the myth is replying NICE
Well, @Roguelab, if you were extracting with -78°C alcohol, chances are you had water condensation dissolving in it, which, as many have said, will form H2CO3 (carbonic acid) in contact with dry ice. The formation of this acid is why taking a breath of CO2 from an open chest of dry ice stings your airway, albeit briefly… as CO2 makes contact with moisture in your airway, the acid is very briefly formed and quickly disassociates into HCO3- and H+ (actually H3O+) ions. This is a perfect example of why a protic acid is only acidic (and pH really only exists) in the presence of water!
Because it immediately and completely disassociates, carbonic acid is technically considered a “strong” acid. It is a strong Brønstead-Lowry acid conjugate of a weak Brønstead-Lowry base (which is actually an extremely weak B-L acid). This acid, ephemeral as it may be, is perfectly capable of remaining in solution with aqueous alcohol and cannabinoid resin, and thereby catalyzing isomerization. However, it is unlikely much isomerization occurs while at -78°C, but instead occurs as the solution is warmed to evaporate the solvent.
Carbonic acid is a very common problem for those using supercritical or liquid CO2 for Cannabis extraction. One of the purposes I have given for using MgO is to neutralize carbonic acid in affected crude resins, making magnesium carbonate (MgCO3).
Lastly, there is generally no “singlet oxygen”, unless you’re talking about photooxidation of terpenes or something like that. ![]()
Hey!
i been looking into carbonic acid for isomerize of CBD.
And found this
A few pieces of research have shown that acetone (an aprotic ketone solvent), soluble in water, could reduce the interfacial tension (IFT) of the water-oil system, e.g., smart-water flooding (chemical-water flow) [14,15]. They suggested that this acetone-water mixture could increase CO 2 solubility and reduce the IFT related to the mutual-solvent volume
The following are published[1] solubilities of CO2 in several organic solvents at 1 atm and 20C Solubilities are given in mL/g:
acetone: 8.2
ethanol: 3.6
benzene: 2.7
methanol: 4.1
toluene: 3.0
xylene: 2.3
heptane: 2.8
methyl acetate: 7.4
diethyl ether: 6.3
For reference, the solubility of CO2in water is about 0.9 mL/g under the same conditions.
CO2 is very soluble in acetone. A mix of water and acetone will reduce the interfacial tension.
Could it be possible to create a solution of acetone saturated with CO2. Add CBD isolate and then add H20 and let the CO2 react with the H2O and create carbonic acid. Everything should be cool down to around 0C and Done i a closed container.
Would this isomerize CBD to THC?
Not at that temp but maybe it s a good acid donor for later on when heat (distillation ) is added
I’m chatting with a guy that says they are doing inline isomerization with ethanol and Co2 in a modified holding vessel. They produce around 80% THC.
I’m going to experiment with a soda sifon:
Create a solution of 9,5 ml of isopropanol (I can’t get hold of high % ethanol) and 0,5 ml H2O + 1 g CBD isolate.
Add this solution to a sifon and pressurise it with CO2. Shake the bottle every day for 1 week to make sure the H2O reacts with the CO2 to create carbolic acid.
Isomerization???
The bottle will be basically inert from O2 and free from UV so hopefully there won’t be that much CBN and high on D9?
Nifty information, @Ozone ! The short answer is: Yeah, you can probably use carbonic acid to intentionally catalyze isomerization of CBD/THC, but only if it is set up properly. It happens by accident in CO2-based Cannabis extracts, rather notoriously… and I suspect this has a lot to do with the extremely high pressures involved, since even mildly elevated pressures + heat (without acid) can cause isomerization products to show up.
Rip man
Well the container will have a pressure of around 40-50 PSI so hopefully that will be enough to generate the carbolic acid and have it react with the CBD while it is dissolved in the IPA.