I would think that the OH from the carboxylic group would cause side products. The AA attaches to the hydroxyl group (that is why an acid catalyst would help) of thc to form thc acetate. Your question does pose a question about cbd acetate. Will the multiple hydroxyl groups in cbd create side products and if so how would one remove it? The catalyst used for cbd acetate probably (speculation) wont be acidic due to the chance of formation of delta 8 thc.
Here’s the structure of THC-O-acetate so that ppl know what molecule they’re talking about. The phenol on the A ring has been acetylated.
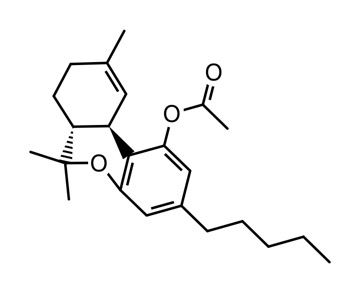
On a side note. I haven’t made this product yet. Does it oxidize like high d9 distillate? If not, that really implicates a benzeno-quinone as the source of the red line people see in their distillate.
no oxidation on sample going on 2 years old
Thanks much. That supports the hypothesis that if pure d9 THC is oxidizing, it needs the phenolic group to do so.
What conditions are you storing the sample in? I believe the reason my sample oxidized so quickly (about a month in I noticed wavy lines appearing) is I didn’t fully neutralize the sample before fractional distillation. After about 2 months the sample turned into the “salad dressing” @Soxhlet mentioned above. I plan to do this again with a catalyst sometime in the future and would like a way to prevent oxidation.
Also while I have you here, have you synthesized cbd acetate?
Yes it’s a catalyst. 2M HCL to remove.
Why did you choose this catalyst over others? I took the time to research triethylamine and it does seemed to be used somewhat commonly to catalyze acetylations at or around room temp.
Could you provide more details about the conditions you ran this reaction at like what temperature and for how long? Were you able to monitor the reaction with TLC or just able to verify with mass spec?
Thanks for sharing!
RT, 5 days, used pentane, should have used heptane to heat. Confirmed with TLC to finish. Nitro blanket.
Produced a decent bit of a unanalyzed product using methods similar to what Skunk Pharm Research utilized. I did ~3:2 molar equivalents of the Anhydride:THC with the THC source being a untested dark brown/black distillate guesstimated at 70%. Distillate(1000g) was dissolved in 2.5L Hexane and acetic anhydride(~300ml) was added. From literature on esterification of alcohols, addition of an acid can catalyze the reaction and increase yields of the esterified product. Conc. Sulfuric acid was used to avoid addition of water and added drop wise until solution was 0.2M H2SO4(Ended up ~12ml 98%H2SO4) All reagents were added with flask in ice bath. Reaction was moved to mantle and carried out under light reflux for about 3 hours with rxn tracked by TLC.
Solution was quenched with water to hydrolyze the Anhydride to acetic acid and stop reaction. Solution was transferred to sep funnel and neutralized with saturated sodium bicarbonate solution(5-10% better, less emulsion), aqueous phase was removed after considerable emulsion separated. Saturated NaCl sol.(again 5-10% better) was added then aqueous layer removed again. Nonpolar fraction was separated and dried with anhydrous Calcium Chloride.
Nonpolar solution containing product was concentrated under vacuum and transferred to short path. Was carefully distilled under a vacuum of 110millitorr. The main fraction distilled at with a vapor temp of 178C. Yield from Main and Useable Tails 718g, theoretical yield was estimated at 793g(acetate is 1.13x larger then THC) however without analysis, yield efficiency is impossible to measure.
Product was pale yellow oil that was much more viscous then d9THC. Was well recieved by those who used it. Without analysis it is impossible to confirm product but is likely the addition of acid caused side reactions and isomerization to a possible d8Thc acetate product. Product did oxidize, slower then THC but after 6 months was a deep red color.
This procedure used significantly less Anhydride then the D Gould method in Cannabis Alchemy. With Acetic Anhydride being harder to get in large amounts, proper catalysis will be important.
For CBD, the addition of 4-dimethylaminopyridine may help selectively produce the diacetate as it is used in an almost analogous rxn to avoid monoesterification. But I have very limited experience so it may cause unwanted reactions(I see you already mentioned this wc15)
Acetylation of the acidic forms should be possible. THCA is salicylic acid with substitutions. Acetylated Salicyclic acid is Aspirin. Acetylated THCA should have very interesting antiinflammatory properties. THCa has a higher affinity for COx2 receptors(common nsaids all work here) then ibuprofen. I take THCA crystals frequently for back pain and it works amazing. The acetylated version should in theory work better
Hi weedaholic721, you bring up a great point! I remember aspirin synthesis in my first year of organic chem. Salicyclic acids carboxylic group wasn’t modified by the acetic anhydride or H2SO4 catalyst due to the greater nucleophilicity on the phenol. This could mean that side reactions would be limited. I wonder if eating thca acetate would partially decarb (from heat and acid from your stomach) it to thc acetate. It could cause either greater back pain relief or just knock you out. I personally found that thc acetate when vaporized provided great muscle relief.
Thanks for sharing!
I saw on instagram that @Kingofthekush420 is trying his hand at Delta 8 Acetate. If your willing to share, what are you using for a catalyst and how are you preparing saftey-wise to prevent acetic anhydrides reaction with water from the air?
I can provide an SOP for this reaction.
I would love to see it! As you probably have read ive been looking for a catalyst to lower the temperature and time of the reaction.
Are you refluxing? Why?
A protection reaction can be completed in as little as 15 minutes. PM me.
I am refluxing a solution of 51g of AA and 10g of delta 9 distillate for 3 hours under a nitrogen blanket/environment. The issue with this is the hot AA could react with the water in the air to form acetic acid which is an exothermic reaction. Without a way to cool the reaction this could get out of control fast. Ideally I would like to find a catalyst so I can run this reaction at room temp so I could motor the reaction safely with TLC.
Again I believe it must be performed with THCa and acetic anhydride. @anon93688 do you think it would work from ∆9 without first carboxylating?
too much bulk and another competing alcohol will make THCa and AA an unproductive reaction. Even with adding a catalyst won’t help overcome this issue.
What makes you think that the carboxyl group needs to be present for the acetylation reaction? We know that the hydroxyl group is highly preferred compared to the OH in the carboxyl group due to the greater nucleophilicity, but I dont see any evidence that the carboxyl group is needed. Phenols being acetylated is a failrly common reaction. The difference I see in using thca vs thc as a starting product is possible yield. There may be more side products if no carboxyl group is present due to that decrease in nucleophilicity.
The temperature of the reaction (unless you know of a better way) is above the decarb temp (50C) so the carboxyl group will removed anyway.
Thank for for replying and contributing!
