What are you using % w/w?
That would be incredible! Since I’ve got your attention, I’ll expand on my current situation. I’m managing a CBD Lab in NC, where the THC regulations are pretty strict at .3% or lower. We are taking Crude to Distillate using Wipe Film Distillation. So naturally, there’s a 2% - 5% spike in THC after the 1st/2nd pass. I need to remediate that while keeping it in distillate form. Ideally, I’d like to keep it simple but we have plenty of resources for R&D. I really appreciate it! If anyone that is fluent in conversion, I might be able to offer some consulting opportunities.
SHOW ME WHAT YOU GOT
Hi! I’ve seen your comments on a few CBN synthesis posts. I would love to see your SOP if that’s possible or take any advice you may have. The reactions I’ve been able to achieve have yielded very low so far. Thanks.
I just joined recently and I would love this SOP! Please let me know
Old thread coming back from the dead
I am curious, whats the gold standard for CBN production these days? I am assuming the Sulfur method has long gone by the wayside for good reason. Palladium and carbon?
Hi there! Came across your posts in a few CBN distillation threads and was wondering if you were ever able to find a decent SOP other than sulphur/iodione/ pd/c. I would be very appreciative if you could point me in the right direction.
Currently doing a heat+UV conversion w/ and aerator at 167c for 48 hours, then distilling through a SPD. Main issue is that there are too many cooks in the kitchen at my lab right now, and I’d really like to nail down a solid SOP so that we can stop collecting all the boof tails and azulene with the CBN ![]()
We recently procured a fraction finder, and I’m in the process of setting that up for our production goals. Any insight you may have into UV spectra of cannabinoids would be amazing, I’ve found a lot of conflicting information.
Unfortunately you’re not going to have much luck detecting cannabinoids with a fraction finder. Differentiating between cannabinoids with a fraction finder is also impossible. It’s not the right tool for that job.
The UV detection you need is much higher energy and the best way is to pair it with an HPLC.
Do you plan to crystallize the CBN? It should crystallize readily in a hydrocarbon solvent which will help you separate any azulene
Thanks for the insight, I appreciate it. Yep, I gathered that the fraction finder wouldn’t be too useful beyond setting visual barriers in the GUI for technicians with even less distillation experience than myself to discriminate between fractions.
Currently I’m struggling with potency, with our best numbers being about 70% CBN by conversion of high potency d9THC under UV + heat @ 167c, run through short path distillation aiming for a vapor temp of 177-185c, but I’m afraid to run my mantle much hotter than 220c out of fear of creating and codistilling some potentially harmful substances. Do you have any thoughts you are willing to share on this? I would like to be able to “rework” the distillate product with additional heat to hopefully degrade any remaining cannabinoids into CBN. Thank you for your patience ![]()
Where are you located? If you have access to high quality HPLC testing that’s where you should start. It sounds like you do based on awareness of your 70% potency.
If you DM me or post here a chromatogram from that LC test I can help start to unpack the issue you’re having. Thank you for the details you’re posting I’m going to go ahead and try this myself
I have a procedure that’s way more mild than 167C, and higher yielding/quality than heat/UV alone. Also, you wouldn’t need an HPLC to run it correct every single time (;
The CBN crude directly out of the reactor from my procedure is pure enough to crystallize spontaneously (>85% CBN), but still should be distilled prior to crystallization.
I’ve been using Pd/C to convert D8/D9 to CBN with pretty good conversion (all starting material consumed). Then I distill and then precipitate but I can’t get above 90% CBN potency. Any subsequent crystallizations yield 88-90% potency. Any one have any suggestions?
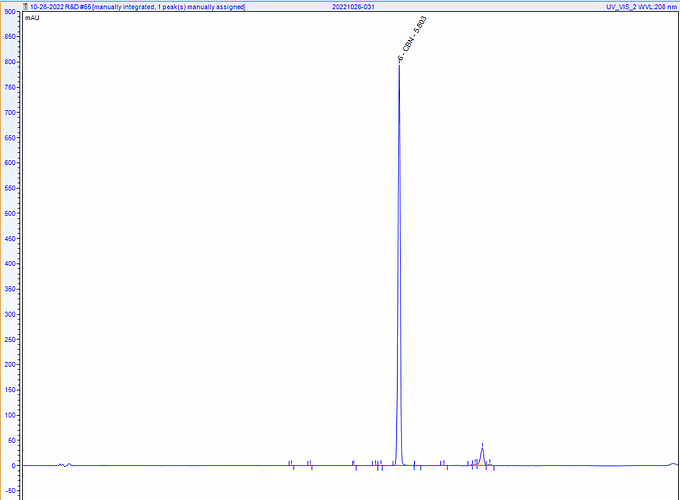
Can try to help. Can you post a HPLC chromatogram of your CBN distillate and crystal?
See here. I think the other peak is HHC but I’m waiting on a standard. In any case, I can get that to disappear but without getting to 99% CBN
Are you able to test the initial CBD isolate to confirm the high end of your expected purity?
The d8/d9 conversion and the cbn crystallization won’t purify trace contaminants in the CBD that initially co-crystallize.
I’ve seen many cbd isolates that test above 90% but below 95%
Why hhc?
You should send out for 3rd party testing to figure out if your contaminant is a THC isomer or HHC because that might help troubleshoot what about your reaction is creating it. If the reaction is clean, the isolation should be straightforward. That said, if you can’t improve the ratio via distillation then your final hope is crystallization - increase the solvent volume and temperature of crystallization to hopefully make the crystallization more selective (by keeping the impurity in solution/solubilized, at the cost of some yield however). You also can use more complicated multi-solvent systems but that’s a whole can of worms.
Why is HHC being formed? I assume that the protons leaving the THC when forming CBN are hydrogenating the unreacted D8/D9 to form HHC. Lab confirmed HHC was formed
Thanks for the advice. I have tried to recrystallize in more dilute conditions but I can try even more dilute. Fractional distillation and then chromatography, in that order, would be the next things to try. The third party lab testing is a good idea, thanks!
Sure I was just hoping people might have precipitation or distillation conditions that have been successful in isolating CBN.
What would be the source of hydrogen?