Question edit: you explained what you meant “after a few scrubs in pentane”.
how long take this crash?
Here is an old trial in a large mason jar
First a crust developed and grew to a degree. We didn’t want it to keep growing so we pushed it underneath into the water
Then we evaporated a bit of the pentane in the mixture and allowed it to crystallize some more. Here is where we ended the growth the second time pushing the puck down
Here’s the crystals all together. Stirring helped to remove the yellow colored mother liquor.
Here are the final crystals. They melt a slightly yellow color which we expect from crystals like this grown in a very yellow mother liquor
Maybe @pdxcanna can help model this using design software
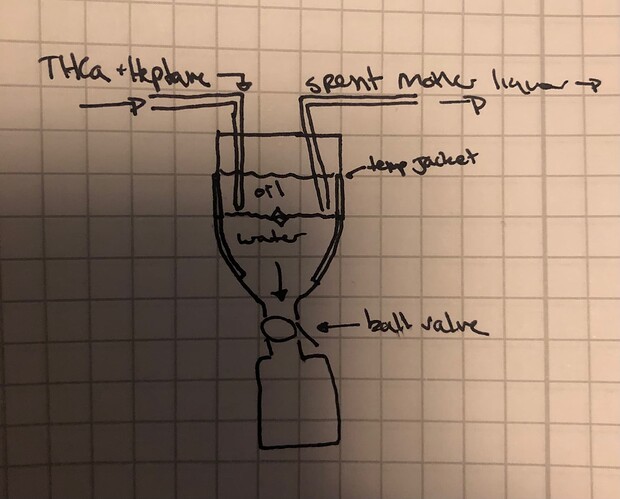
This design allows for continuous crystal collection from the same vessel. Collection is below a valve, which is left open during crystallization. After the crystals are in the bottom collection, the ball valve can be closed so that the layers above remain consistent
Trying out your technique and see what i can do with it, i used heptane and a bunch of sugar so waiting patiently to see what happens.
Wow, @moronnabis & @AlexSiegel ! I absolutely LOVE the way you are overthinking this! I read this entire thread, which is very rare for me to do with long threads with meandering topics these days! Highly enjoyable! I had several responses to various statements and questions you both posed in your discussions, but I believe you recognized it was (almost) a purely academic exercise. I missed it while it was happening, and I don’t have the time to go back over every line the way I did, already, but then try to respond on this admittedly glitchy mobile browser display of the forum!
I just wanted you to know I appreciate your thought processes! ![]()
Also, back on topic, @AlexSiegel , you’ve done the community a great service by demonstrating and explaining your experiments in bilayer interface crystallization of THCa! This technique introduces antisolvent concepts, phase theory, the interplay of hydrogen bonding and Van der Waals forces, as well as a host of other useful phenomena in crystallization practices! Thank you for pulling it out and brushing off the dust for everyone to see it shine, my friend! ![]()
What do you think about agitation and/or ultrasonics in this process?
If THCa really isn’t very soluble in butane wouldnt that mean you could push it out of solution by dissolving something really non polar into the butane after extraction?
In which process, @tweedledew ? I would only advise either of those modalities for homogenizing mixtures of ingredients. Agitation must be relatively gentle, and only used on a saturated solution of “strongly” precipitating crystallizing solutes for the purpose of achieving a relatively narrow particle size distribution… i.e. making rather tiny, high-purity crystals all about the same size (usually for ease of filtration and high uniformity across batches). Ultrasonic energy is generally far too powerful a source of energy for a negative-entropy process like crystallization.
You don’t think you could take a hydrocarbon saturated with crude and then by quickly chilling it and applying ultrasound to induce crystallization?
Sounds like it’d work
Sonocrystallization is what it’s called I think.
Excellent point, @Franklin ! Honestly, the folks in here (including your earlier post) have all hit upon the salient causes for THCa’s solubility in butane (and the like), but I believe they were intentionally ignoring them for the sake of exploring unknown possibilities, as well as further elucidation of the subtle mechanisms of solubility. ![]()
My last brain cell couldn’t keep up with their jargon.
I hear ya! I believe the major gist was asking “Why does this ionic molecule, THCacid, dissolve so easily in non-polar solvent like butane, and at such cold temperatures?” And “What if it’s not actually THCacid, but instead it only exists in the plant resin as the conjugate base, the THCcarboxyl-anion (THCa, sans H+)?”
They said something about salts of THCa, how are those produced?
I was thinking low speed agitation to increase crystal precipitation, and to better clean/tumble the settled crystals at the bottom.
I guess maybe if you had a large enough alkane Layer you could throw a low impedance sonicator on it and not mix the two layers but rather just rattle the alkane one around to spit out some crystals.
Just a stoned thought, no pun intended.
This is an in-line ultrasonic device I use for milling CBD and nucleating it. Or sometimes making emulsions.
Maybe there’s something that could work with the two concepts?
XD yay thca ![]()
![]()
![]()
appreciate the note:
Yes, have you ever wondered why they like to run “sub critical”
with up to 35% EtOH… maybe the supercritical just doesn’t have
the butane “juice” aspect.
Oh a theory about the “juice” !
beware the “jargon”: solubility enhancers
aspect:Drug Solubility: Importance and Enhancement Techniques