We are working to develop simple GC methods for measuring cannabinoids in edibles like gummies.
Our current preference is to dissolve the gummy in 10ml of hot water.
Then we add 10ml of hexane or heptane ( with an internal standard ) to the vial and shake for 1 minute.
When the phases separate we get water on the bottom, a stiff gel layer in the middle and a small heptane layer at the top. We inject the top heptane layer and appear to be getting good results.
I would be grateful for advice from others who have developed similar or better solutions.
Hugh
SRI
I’m using water too - with a standard Quechers separation for my injection.
I’ve also seen people use ethyl acetate - when they freezer and grind the gummy using like PTFE coated bb’s for the grinding in the test tube.
The AOAC method for most “foods” says to use quechers when you are trying to separate the aqueous from the organic layers. Its worked for me. The specific method is for pesticides… the sample prep works just as good for potency, IMO.
How expensive and time consuming is Quechers. Seems like quechers is good for pesticides on strawberriers but overkill for cannabis in gummies.
Do a further wash of your heptane with methanol. It’ll reject even more gunk. Although it certainly makes it more time consuming.
Acetonitrile would have a similar effect.
It’s really not very tough and it is worthwhile
Quechers : “quick, easy, cheap,…”. The water method you are using works just fine though, I would advise Quechers if you are going to MS, candlestick is less of an issue. You may save yourself money on inlets and needles.
Not familiar with the “candlestick”. What is that.
Hugh
Are you sure some of the cannabinoids would not partition into the methanol.
Hugh
I should’ve been more clear.
The heptane will hold non polars (fats/waxes) the methanol should hold primarily cannabinoids. So your heptane layer would mostly just be contaminants after the methanol wash.
If the cannabinoids are decarbed rather than the acid forms ( which you would expect in a gummy ) why would they be more soluble in methanol ( polar solvent ) than heptane.
Just slang for FID.
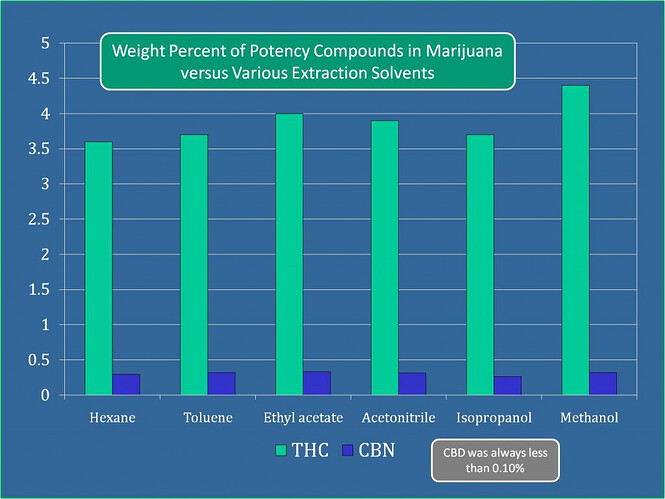
There’s a few posts on the topic. But methanol has nearly the highest affinity for cannabinoids than any other solvent we’ve tested. If you mixed an equal part heptane and methanol solution with cannabinoids, you should notice a greater concentration of cannabinoids in the methanol portion.
I don’t have a chemistry background, so I can’t explain the mechanics. But it’s certainly been noted around here
Yes some of them will. Unless you are controlling for pH (which is what quechers does). quechers is cheap, uses water and ACN - and won’t carry over sugars, fats, etc - that some of the other Liquid Liquid separation techniques do.
You want your separation solution to be basically the same as what you are running on your machine as well - so you don’t get wonky injection peaks (you can always do extra stuff to not care about them, but easier if you are using chemicals that expand/heat at the same/similar rate).
Quechers is designed to be cheap. Its designed to pull out all the bits that are water soluble - then using pH control only push over into the ACN what you want. It works rather well for cannabis and for pesticides…
I’ve been testing edibles for about 5 years now - I’m not trying to make your life harder. There are people who give no fucks and will just dissolve everything in MeOH. You can do this, but will have crap repeatability and inconsistent recovery. Plus you’ll be having to change your column more as you fill it up with gunk. Columns are expensive - quechers is cheap.
Check out this paper that even discusses this. ![]() I’m gonna say its because of the partial positives in the cannabinoids which are attracted to the negatives in the MeOH. These guys know what’s up - because this is what they do, design columns so you get the best recovery. Looks like @Chaboes also posted this.
I’m gonna say its because of the partial positives in the cannabinoids which are attracted to the negatives in the MeOH. These guys know what’s up - because this is what they do, design columns so you get the best recovery. Looks like @Chaboes also posted this.
You can do it different ways. The ones that have done it the best… they are getting better than 90% recovery (meaning they don’t leave stuff behind after trying for separation) and they get consistent results (meaning they get less than 2% variance when prepping the same spiked sample over and over again).
The method in the link is for FLOWER - so you need to consider the solubility profiles for the other ingredients in your edibles. Following on with Quechers - will allow you to remove all the “food” from the sample and just keep the stuff you actually want to be looking for.
I’ve used it successfully for cookies, brownies, peanut butter cups, gummies - gelatin, gummies - pectin, and flower. I did have issues with it for artificial chocolates, but not from natural cacao products.
If you don’t want to use this method you can try the following methods.
- Flash Chromatography - testing fractions until you find what you want.
- Doing nothing at all - just prep like you would flower, and use a syringe filter + pre-column on your instrument (be prepared to replace that pre-column every 100-150 samples)
- Cryofreezing your sample, grinding it up, and then while everything is still below the solubility for the other ingredients in your edible, pull over the cannabinoids (this won’t work if the temperature is below the solubility temperature of cannabinoids, and you will have lower recovery).
But really - use a method that others have used before. Here is a more specific to EDIBLES for cannabinoids, instead of edibles in general, if this will make you feel more comfortable.
You could also use a lab that already does this. Buy a method from someone that has already done this. Or hire in someone that has done method development before. I’d recommend hiring someone if you start trying to do “special” or “novel” edibles.
Good luck!
Hey thank you for your information! I’ve been using the water separation and quenchers with acetonitrile. After the separation and syringe .22um filtration, I use a 1:1 IPA, MeOH for dilution for analysis. Sometimes I get extremely accurate results and other times I get things 15% above expected. Do you think there is anything about the density of the ACN in 1:1 that the pipetting density for a calibrated 100uL would be an issue? Just a shot in the dark, might just be a pipetting inconsistency. Let me know what you think if you could! Thanks!
have you sat with a scale and investigated that? well worth 10min to check. certainly a thing with new pipetter users. or dirty pipetters (often ALSO a new user thing)
It doesn’t seem to be an issue with any other testing methods we have for tinctures or topicals, also similar dilution solvents, but larger sample inputs for first dilution. I’ll check on that scale suggestion! Thanks. I do accuracy checks on the pipettes weekly and they are well kept, so I don’t believe it is an equipment issue. Between the tincture/topical testing and this there seems to be a variable. Would you think a dilution with only MeOH would be better than a 1:1? It’s been a while since my university chem years.
no idea.
it might even be that what you were taught and what actually happens are different.
I think I was able to figure out the overall issue, but it seems like the ACN separation in the first phase wasn’t returning a perfect 10mL. In some cases it was 1-2mL less which, across results, definitely seemed to correlate causing an over concentration of that being a first dilution leading to higher test results.
Do you take an aliquot of your ACN and dilute?