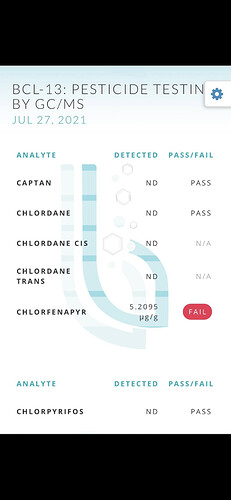
So I feel like this is a super dumb question, and feel like I should know this, but Is it possible pesticides can be recovered with the gas? I just ran some fresh frozen from a grower we don’t really know and it failed for pesticides in a huge concentration. He swears he doesn’t use any pesticides and has never heard of what we tested positive for. We ran some other material from a grower we know had some pesticides in it a week before running the fresh frozen. I didn’t clean out my solvent tank in between and am wondering if the first run may have contaminated my tank and solvent which then made the fresh frozen fail testing. I can’t really find any info on if pesticides can make it back into the solvent tank along with the gas being recovered.
What’s the pesticide, and what’s it’s boiling point?
Did you test the dirty by chance?
Unfortunately no
Test your solvent, I’ve had certain pesticides stay in before. I’ve yet to have to deal with chlorfenapyr. There maybe a magic powder that will solve this for you.
Here’s a list of possible pesticides it could have come from
Ya we’re pretty sure it was phantom. But can phantom be boiled off and mixed with the hydrocarbons when recovered?
hey brotha we deal with lots of farms that pop for clorfenapyr. Most old warehouses have been treated with bug bombs that contain clorfenapyr. this stuff gets in every ac duct, every crevice and any insulation in the grow. My buddy has changed out every piece of equipment and still pops. Finally he sealed his insulation in the attic where the make up air was pulling from and the levels dropped. He had his whole facility swabed to see where the concentrations were and the whole place popped but heaviest around ac units. Most farms that pop for this do not use or have ever used it but their grow is fucked. I know a few huge growers dealing with this issue. We also have not found any remediation for clorfenpyr. That being said I have run stuff thats 100% positive had 10x limits of clorfenypr and the very next run came back clean. It doesn’t seem to stay in the solvent. We didn’t do any special cleaning of the tanks as we had done tests In the past when we delt with this issue.
Good luck explaining this to the grower as they always want to blame us. Their flower will also pass even if the concentrate failed 30x. There are a few labs that dont catch clorfenypr but any good one will catch it every time.
Also Its used for mosquito abatement. I have a conspiracy theory the state sprays this shit everywhere near bodys of water and It gets tracked into grows and it also carries in the air for miles on a windy day. Almost how the state used to use broad and russet mites as weed abatement tools and would release them on the sides of the 101 freeway and that shit would catch on your clothing if you drove with the windows down and get into your grow.
Some counties in Michigan do this, I wonder what death liquid they are spraying. You can’t drive 5 min in most parts of Michigan without passing a body of water. So to think it’s not getting in the water would be stupid. ![]()
country boys be like “got to prevent the west nile and other skeeter dispersed disease, so we goin to pesti bomb the watershed with Pylon”
Yea unfortunately its commonly found in watersheds as it was used to prevent mosquito viral transmission, reduction of bed bugs, thrips, gnats and mites (only approved on non food crops EPA Fact sheet), instead of fostering healthy populations of Beneficial insects, swallows and bats.
According to its Safe Data Sheet, chlorfenapyr is “very toxic to aquatic life with long lasting effects”.[5]
adsorption and occlusion as potential solutions
As it is water soluble, and hydrocarbons can carry some H2O, there is a chance that the gas can become contaminated. test the Gas, it may require replacement or passing it over a selective adsorbent ( homework time : Adsorption chromatography)
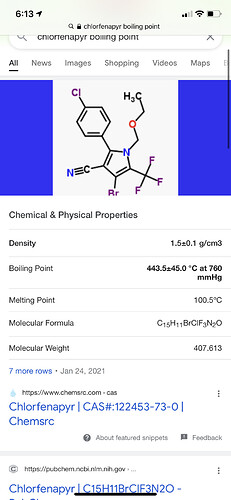
As its BP is just above the target APIs, it may be removed using fractional distillation, though this may be a touch difficult unless you can really accurately control your temps.
alternatively, I would recommend developing a method for a LLE to allow the pesticide to transfer into the aqueous phase, with the target API left in the organic phase. that’s what they do in the veggie oil industry, and more recently, there are published patents stating this can be done using liquid—solid adsorption chromatography (LSAC) within our crop sector:
"11. A method for using bentonite to remove pesticides from an initial oil extract of cannabis , and wherein the initial oil extract comprises pesticides, wherein the method comprises scrubbing with bentonite, wherein the method comprises the steps of: (a) Mixing the initial oil extract with water and acidic water in order to remove water-soluble material from the initial oil extract, thereby producing a processed oil extract, (b) Adjusting the processed oil extract to a pH value of less than pH 3.0 or to greater than pH 12.0 to increase ionization of nitriles or pyrrole groups or of both nitriles and pyrrole groups, (c ) Mixing bentonite with the processed oil extract that had been adjusted to a pH value of less than pH 3.0 or greater than pH 12.0, (d) Allowing pesticides to adsorb to the bentonite, and (e) Separating the pesticide-depleted oil extract from the bentonite, resulting in a pesticide-depleted oil extract."Methods for Using Bentonite to Remove Pesticides from Cannabinoid Extract Oils
Solubility of
Chlorfenapyr.pdf (fao.org)
in organic solvents at 15 to 25 °C a
acetone 133 g/100 mL
acetonitrile 53.7 g/100 mL
dichloromethane 169 g/100 mL
ethyl acetate 78.6 g/ 100 mL
hexane 0.69 g/100 mL
methanol 5.13 g/100 mL
toluene 71.5 g/100 mL
Not very soluble in Hexane or water, more soluble in toluene dichloromethane acetone
Boiling point Decomposes before boiling a c CK-334-001 EC Annex V method A.2
Decomposition Temperature
183 °C, onset of decomposition a c CK-334-001 USEPA OPPTS 830.6316
Photochemical
degradation
Chlorfenapyr is rapidly photolysed in water a f
t½ = 5.2 days (pH 5) (average) Lower looks better…
t½ = 7.5 days (pH 7) (average)
t½ = 4.8 days (pH 9) (average)
I was able to find a paper looking at remediation of it in soil & water, Green nano-phytoremediation and solubility improving agents for the remediation of chlorfenapyr contaminated soil and water
based on its structure, might be a target for myco-remediation as well.
What pesticides did you have stay in your solvent?
I’ve heard @Killa12345 tell of this I think… I remember him calling it hot solvent then saying he’d let it all go if any biomass was in questions