Can this be done with distillation? Or is it just better to use a catalyst that make little to no iso-THCs? I’m looking for an option besides chromatography to make high purity D9/D8.
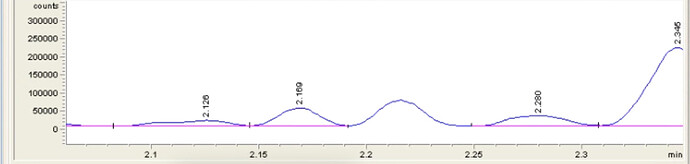
Well, I’ll tell you after I run some samples afterwhile. I’ve been meaning to run my heads, hearts, and tails for a while to see what the chromatogram says. I’ll see what I can post up when I get some samples run. I do not have iso or exo THC standards, but I have noticed the “penis peak” as we call it that elutes before the CBD in my GC-FID column. It starts as a lil penis and grows as the RXN continues.
That would be awesome ![]() I think we’re working from the same patent where AlCl3 is used to produce a 10:1 ratio of D9:Iso-THC. I’m surprised (not) that this topic has received less attention then remediating d9 from d8 since the safety and effects of iso-THC are totally unknown (especially when the process of remediating d9 often produces lots more iso-THC
I think we’re working from the same patent where AlCl3 is used to produce a 10:1 ratio of D9:Iso-THC. I’m surprised (not) that this topic has received less attention then remediating d9 from d8 since the safety and effects of iso-THC are totally unknown (especially when the process of remediating d9 often produces lots more iso-THC ![]() )
)
Yes! Exact same elution spacing! We were running more concentrated with our RXN sampling and it spiked up like a penis!
I regret that I got a wild hair up my ass to deep clean the shop today for “Friday Reset” and i wielded a squeegee instead of my sample vials, I will most certainly get on this next week to show y’all! We always giggle when we see it come through.
If you want to make very pure d9-thc then the best way would be to stop the reaction before any d8 and iso is formed, because it is easier and better yielding to seperate thc from cbd than thcs from each other.
Would someone like to share a simple, cost effective chromatography routine to seperate thc and cbd, please?
Centrifugal partition chromatography (cpc) is the most economical way I’ve seen it done. But still eats lots of solvent, just not as much as flash chromatography. Can reuse the mobile phase solvent but it’s lots of work to do at scale. And the cpc machines are not simple to operate. This is in reference to cbd and thc separation, probably cpc would be useless for thc/isos
Does TIBA and BF3 produce iso-THC as well?
Is it similar ratio of 10:1 D9:iso-THC?
Why is iso-THC produced at the same time?
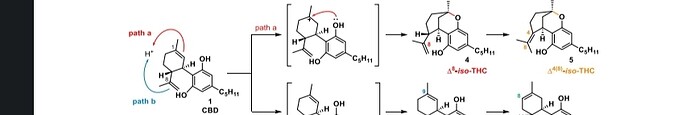
The iso-THC forms at the same time as the CBD because it is just the resorcinol reacting at the other olefin in CBD. It’s a minor pathway but impossible to avoid. It forms with BF3 or iBu3Al but the ratios may change depending on temperature, solvent, reaction time, etc.
If you chemically convert CBD then iso-THC will form despite your best efforts. I would bet that it carries over with distillation, chromatography, or counter current. The iso-THC fingerprints are likely permanently stuck with that D9 material.
The only conversion of cbd where no iso-thc is formed according To the paper is CSA in toluen but the yield of d9 thc is low .
That paper relied on NMR to determine the % of iso-THC because they had complete coelution by HPLC. If GC had been used for the CSA conversion they would have found iso there as well.
I have found, in my experience, the iso-THC pathway is favored at lower temperatures. I can tell you that EtoAC allows for the production of more ISOs than hexane which also produces more than heptane. This problem is difficult to optimize especially when producing d9 because d9 is lost to d8 with excessive heat, while ISOs are produced less with excessive heat.
CSA forms more iso than PTSA (15% versus les than 5%)
Iso thc is formed when the alcohol group is protonated. This is more likely to happen with a strong acid and higher temperatures.
Wish @mitokid was still around this is one of the many conversations weve had
In neither of those mechanisms is the alcohol protonated. The difference is just from which double bond gets protonated.
Yeah man, not trying to knock you, but I have it on not only good authority that higher temps help to decrease ISOs but as well with in house GC-FID with upgraded column to differentiate CBD from ISOs.
It apparently has something to do with the nonplanar structure and the angle between the aromatic ring and the phenolic tail. Allegedly this angular difference comes into play at lower temperatures. Please correct me if I am mistaken from our conversations @kcalabs
I can literally show you reactions ran under the same conditions that at higher temp produced 5% iso and at room temp produced under 1%
Would love to see it, brother! I’m not authority. Just done a metric fuck load of test RXNs
So have I and all the cleanest highest testing reactions were done at room temp
The higher the temperature the more exposed the double bond is due to thermal expansion. This is also why when you make d9 you get more d8 at higher temps because the double bond is more exposed due to the molecule being bigger
Distillation can be used to separate d8 and d9 THC, but it might not entirely eliminate iso-THCs. Using a catalyst to minimize iso-THCs is a good alternative, but it’s crucial to ensure the process is safe and efficient.