I think they’re rexing in pentane, but with a dramatic loss in yield
I have made plenty of diamonds in iso back in the day. I don’t think it’s the culprit, although the butane is reacting as iso would at room temps but iso still holds more solubility at same temp in my experience
I think that with slow enough diamond formation, the problem goes away.
It is generally accepted that for any crystallization, slow growth affords less crystal defects and less solvent inclusion.
There are ways to counteract the crash IMO
I was reading there are parameters to avoid/follow. Forget which thread, but involving the pour
Does that mean it’s evaporating during the pour before becoming trapped in soltution? I’m just curious, this whole thing is something else, and I really for everyone losing money
Dumb fuck here, signing off. I’m too dumb for you guys. Sorry I even try. I’ll just roll over and die. Feed me to my dogs.
Bro, stop that. You’re not dumb or stupid. I don’t think it’s a competition, and you’re kind of making it one. Not being a jerk here, and I respect you. You know this
Ima buy some propylene and experiment with it. I noticed the jars that do this have a weird odor as well
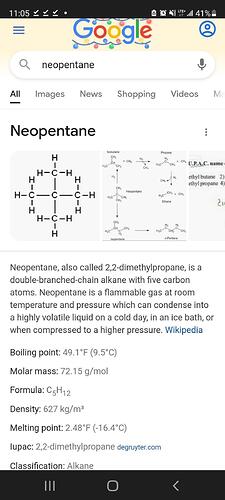
Neopentane too. Propylene is a much higher pressure than butane. One thing I dug into was seeing if the tanks were lp239 or lp260. If they were the 260 tanks, they could have been used for propylene before shoving butane into them.
Neopentane would explain a lot of the issues were having, too. It’s melting point is pretty high, which could explain why everything drops out at such a low temp, as it’s turning into a solid and grabbing nearby compounds in conjunction. Usually first being a bunch of fats, which I know I’ve had that issue, and then after the pour causing the thca to interact in an abnormal manner. And it’s BP is above the temps of cooling butane where it could easily still hang around in a concentrate rich solution, still attaching to the lattice as they are forming.
If your rite this points back to the gas supply & we can not deny that demand for butane and the like has increased exponentially in the last 5-10 years, giving way the the idea that refinery’s have had to step up their production to meet our industrial demands.
On a side note, I have also not yet seen or heard of this issue anywhere in Canada, where labs are legit, coast to coast using butane. Gasinnovations seems to ship alot of butane to lab suppliers here in Canada & I have yet to see a medusa stone in my lab. Diamonds is just about all we make really
2¢
![]() Ive only ever had a bump on a residual solvent test result for propylene when using pentane to crystalize, am I alone on that one? just lucky maybe ?
Ive only ever had a bump on a residual solvent test result for propylene when using pentane to crystalize, am I alone on that one? just lucky maybe ?
@Diamondalchemy when you crack a jar and it sits there completely still even though it has tons of butane in it then it spontaneously and forcefully ejects from the jar. I’ve seen some jars just sit there for minutes then just rocket oil out of them everywhere.
I learned my lesson three different times with that. Quite the slap in the face. I retired a pair of shorts (not underwear) because of it getting back splash all over them trying to contain the eruption.
This description of neopentane lines up with what John said about the diamonds not redissolving easily
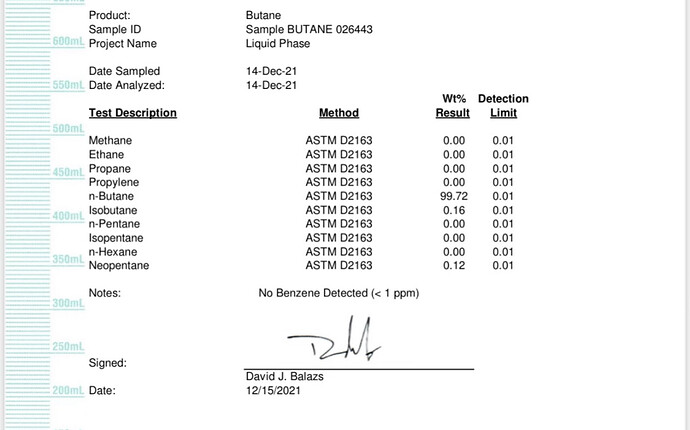
Here was a brand new tank sent to me to try. Waiting for our results of our trial run and will report back. I see neo pentane in every coa I’ve been given of gas.
@Waxplug1 its not the material. The first diamonds to show signs of chalking were from a friends garden who hasn’t changed his recipes in years. He was my first phone call and I was bummed when he said he had changed nothing because I really wanted the blame to lie with the material.
We have sent off samples of diamonds pre and post chalk ans some mid chalk and the results are all the same. Thca and nothing else.
Here were some of the last to chalk up. Had tried a few things and wanted to see if our old sop would work. Sure enough it did not.
Yea I see now it does appear like neopentane is what’s being trapped in the lattice. Anyone know where to source some neopentane to try?
must be a stateside supply available
You could crack open a chest freezer
You’d think it’d show up in solvent residual tests of the prechalked stones, no?