So I have been using a 70/30 butane/propane solvent blend because I’ve always had the best results with this blend, it seems to pull a decent combo of terps and cannabanoids from a wide range of materials, and due to the higher pressure of the propane I have not needed a nitro push to achieve decent flow rates thru my crc.
I also refuse to scrape so I always leave a small amount behind to be able to pour without too much loss on the sides of the platter.
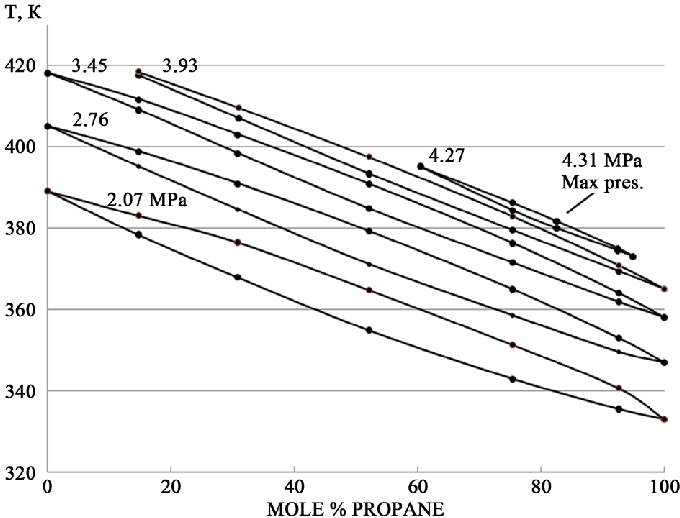
I have been using the same 50# solvent tank (24lb capacity) to inject and recover into, and as most of you know when using blends and pouring, you lose some butane each time because the propane boils off first, making your solvent tank become more propane rich each time.
Well, this is becoming a pain in the ass because I have to adjust my ratios every 3-4 runs, and right now my tank is around 80/20 propane/butane…. Planning on getting a 100lb jacketed solvent tank to use as my injection solvent tank, and use this smaller tank to recover into, so I don’t have to adjust ratios and can just run the same blend from the main tank until it’s gone, as long as the ratio in that tank will remain the same even when its not full.
But I’ve been sitting here thinking about the best way to go about removing the propane from this tank without just off-gassing it so I can do another run, and I had a few ideas, but I don’t want to attempt anything without hearing some feedback on them first.
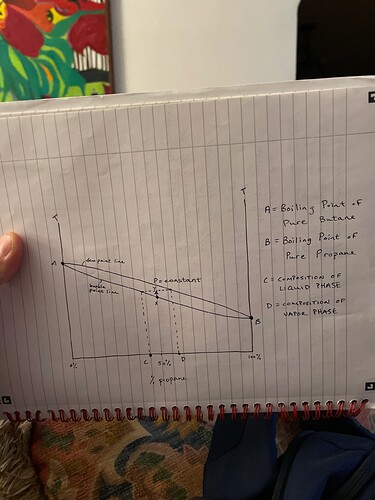
So, first question, if I were to want to just remove the propane from a blended tank that was say, 50/50 propane butane, could I theoretically take an empty tank, vaccum it down and place it in a DI slurry, and then connect that to the vapor port of the tank with 50/50 in it, place that tank in ice and let it condense the butane to the bottom(not sure how long this would take, maybe someone knows or has a formula?), then open the valves and transfer just the propane to the receiving tank? Since the propane will still be a gas and sit on top of the liquid butane? Or would that still transfer butane also? Would it help to have a condensing coil in DI between the tanks? Maybe use a Recovery pump to aid the transfer?
Second question. If this does work, would I also be able to transfer the extra propane to a regular propane tank from the gas station this way and then use it to grill with?
If that’s a stupid idea, tell me, but I’m just spitballing for ways to recycle and repurpose this extra propane. Could also store it and use it for warm solvent pushes thru my injection coil, or mixing with straight butane to create a blend when I refill my main tank…. trying not to waste it if possible.
Just wondering if anyone has done something like this or not, and if this is a stupid idea because of A, B and/or C, or if I stumbled on something that we could potentially use to improve efficiency
And I have looked all over the forum and the internet for a week trying to figure this out but I have not found a clear answer on the topic yet, so I decided to start one.