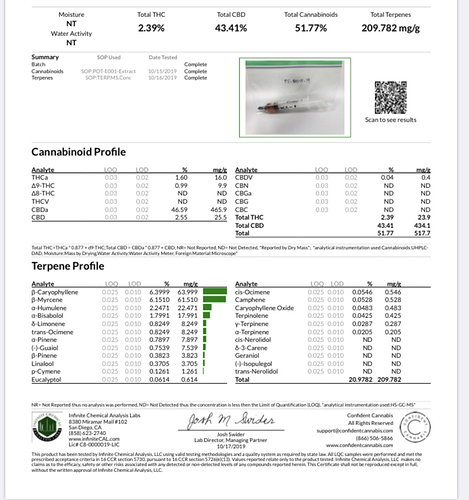
been trying to find more info on these types of systems. This was a great thread. I dont have experience using one, but a colleague ran some of our material through a r134 system here locally, and I was so impressed with the full spectrum oil that he made. Hence why i am trying to find more info. As was previously mentioned, we saw the same results. non-decarb produced really low yields, but the terpenes were incredible. I think it was 41% CBD and 20% terps. debarbed before extraction (which wasnt done well) was almost double yield, but terps were down to 7%. I never ran a residual solvent test, but trying to get some more to run that.
Awhhhhh hahaha haha has someone decided to try making diamonds with r134a concentrate !!!
I’ve been tracking the HFC 134a also for at least a year. Only thing I will say is that there must be ZERO residual — the bad thing about 134a is that it is deathly toxic when combusted. Deathly. ![]()
Very interested however in terp fraction which is why i think there is useful application
Did you ever have any documentation or coa regarding the 20% terp pull and profile? That would be great to see.
Your TAC is fairly low
Was the biomass low in cannabinoids to begin with
Or are some left behind ?
Great point. I forgot to mention that. This was some early material so it wasn’t fully matured. However total cannabinoids going in was 12.4%. But the the spent material was testing 10.6% total after extraction. So there was a lot left.
From the simple tests I have done, the solubility of cannabinoids in R-134 is very low.
With ethanol or hydrocarbons you rarely run into the solubility limit but with R-134 you are going to have to extract with many bed volumes to get complete extraction.
So basically very poor quality back yard BHO is easier, cheaper, and if everything goes wrong you get killed.
And with r-134 extraction is more complicated, more expensive and if you fuck it up you could hurt or kill a bunch of other people.
Pretty much.
Same is true even with water hash. Catch that salmonella or ecoli and kill some people by food poison and dehydration shitting themselves
Gotta be on point
Do you think this would be a viable anti solvent for crystallization? Maybe in a binary solvent system with propane ? Maybe @RockSteady or @downtheterphole can chime in
these guys say terpy and very poor extraction unless decarbed. sounds like a very good candidate.
I’m thinking propane and not butane because the propane will boil before the r134a
might be worth a shot, its potential high terp affinity/low cannabinoid affinity would hold impurities, and help “squeeze” out cannabinoids of the primary solvent, give a shot starting at 5% r134a, scale up the ratios from there!
you bought that whole plant for like $4M+? Has it been installed and started up??
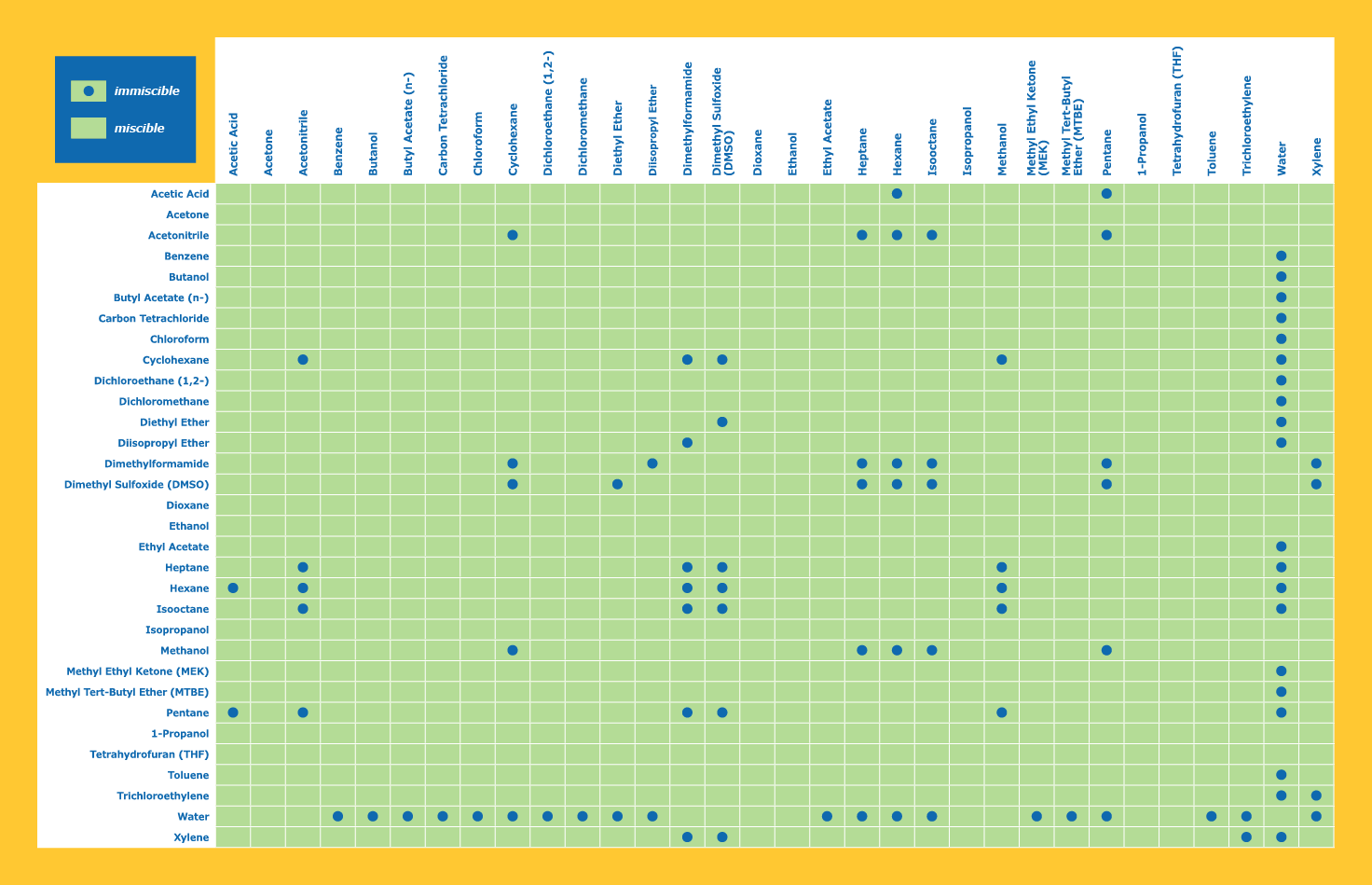
Can someone post a miscibility or solubility chart for 1,1,1,2-Tetrafluoroethane aka r134a
Or maybe just add it to this chart
To be clear I’m not doing this and I’m not endorsing this. I don’t know enough about how this solvent behaves.
Way late to the party, but I recommend jacketed extraction vessels for this reason.
My biggest question is, will combining a hydrofluorocarbon with a hydrocarbon contaminate the hydrocarbon indefinitely? Maybe some sort of unbreakable azeotrope or even a chemical conversion…