Figured I’d share a nice way to accurately test the acid content of an extract called an acid-base titration. It can’t test for the decarbed cannabinoids and so won’t give an accurate overall THC content (although if it’s from fresh/frozen, the number will be pretty close). Its still incredibly useful though, I do a quick titration after every extraction and based on that number I decide if theres enough product to bother extracting that materiel again, or if it’s tapped out. Saves à lot of wasted time and product. Also useful when doing a wash with an organic/aqueous layer. You can actually titrate each layer and from there decide if either later needs to be topped up in order to get the ideal ratio of lipids/salts in each layer, again saving time.
I’ll briefly explain the theory before the walkthrough. Mostly because I’m just a big chemistry nerd and I like getting into the weeds with it, but also b/c I can’t stress enough how important it is to have at least a basic understanding of the underlying chemistry. I was a backyard chemist long before I went to school to become a real one (ever since I stumbled across a cocaine extraction from coca leaves tek back when I was a teenager lol), so I know first hand how frustrating it can be following an online tek and coming to a dead end and not having any idea what went wrong (as something always does). A basic understanding of the underlying chemistry so you know what was supposed to be happening will go a long way towards being able to troubleshoot the issue. Also, with this tek specifically, you’re going to want to be able to customize the measurements/parameters to adjust to your specific extract and you need to know what’s goinig on behind the scenes to do that. So here goes.
The theory behind a titration is simple enough: A basic solution is added to an acidic solution (or vice versa), and neutralizes it, always in a 1:1 molar ratio. Strong acid/weak base, weak acid/strong base, strong acid/strong base, weak acid/weak base, it doesn’t matter… they all neutralize each other on a mol-for-mol basis. So if we have an unknown amount of acid in a solution, but we have a known molar amount of a basic solution (because we created it), then it’s easy enough to figure out how much unkown acid we have simply by counting how many mols it took to neutralize and then mulitplying by the acids molar mass.
For example, imagine we have an unknown amount of sulfuric acid in a solution. We create a known concentration of potassium hydroxide, titrate the acid, and find that it took, say, 0.78 grams of KOH to completely neutralize the acid solution. The molar mass of KOH is 56.10 g/mol, and so we know that it took 0.01390 mols KOH in total (0.78 g KOH/56.10 molar mass = 0.01390 mols KOH). And b/c we know acids and bases react with each other on a 1:1 mol basis, we then know that there must have been 0.01390 mols of H2SO4 (sulfuric acid) in the acid solution. The molar mass of H2SO4 is 98.079 g/mol, so we then know that our unknown acidic sample contained 1.36 g/sulfuric acid. (98.079 g H2SO4 x 98.079 molar mass = 1.36 g). From that information, we can then quickly find out our qualitatitive and/or quantitative information. For example, if we’re looking for concentration, and the volume of the acid sample used was 10 ml, then we know that the sample (and thus the larger batch the sample came from) has a concentration of 13.6% w/w (water has the convenient w/v of 1 ml = 1 g, so you just divide the weight of the acid by the volume of the water and mulitply by 100 to get a %, in this case: 1.36/10 = 0.136 x 100 = 13.6%). And if we’re looking for total mass, then you take your concentration (1.36 g/10 ml = 136 g/L) and multiply it by how many L of solution you have in total. If we have 923 ml of acid solution, we know that we’ve got a grand total of 125.25 g/H2SO4 (136 g/L x 0.923 L solution = 125.5 g H2SO4)
Also, don’t get stressed about “mols” or “molar mass” or “equimolar” or any of those fancy chemistry words. “Mols” is simply a specific number of a given thing, the same way that a dozen is a specific number of a given thing (12). It’s just a really fucking huge number (6.022 x 10^23)… It’s so huge that only in chemistry does it have any practical use, but theoretically, just like you can have a dozen eggs or a dozen cars, or a dozen basketballs, you could have a mol of basketballs, or a mol of eggs, or a mol of cars etc. They’d take up pretty much the entirety of space in the known universe, so you’d have a hard time parking them all, but you could do it. Anyways, understanding mols is super handy in chemistry b/c almost all chemical reactions occur in nice round mol numbers. Not all are 1:1 like acid-base reax are (for example, the reaction that creates carbon dioxide is 1 mol carbon + 2 mols oxygen, and hence, CO2) but almost all reactions occur in nice, round, molar numbers. 1:1, 1:2, 3:1 etc etc. You won’t ever see anything weird like 1.2:3.03 or anything, thank god.
So when you see the molar mass quoted in a number like, say, “123.45 g/mol” all that means is that if you had 6,022,000,000,000,000,000,000,000,000 of that molecule, it would weigh 123.45 grams. Now that you understand that, you can see how this can be helpful when you’re doing your extractions: The molar mass of NaOH (the base we’ll be using to react the acid from our extract) is 39.997 (if you were so inclined to look it up, you’d find that 1 mol of Na is 22.98 grams, 1 mol of O 15.99 g, and 1 mol of H 1.01 g, for a grand total of…you guessed it…39.997), and the molar mass of THCA is 314.45 g/mol. Since vwe know that 1 mol of NaOH will react with 1 mol of THCA, what that tells us is that for every 39.997 g of NaOH we need to neutralize all the acid in a given solution, there was 314.45 g of THCA (unless you had a high CBD extract, in which case you’d use the CBDA molar mass). Or to simplify that a bit, for every 1 gram of NaOH is required to react all the acid in an extract, there is 7.86 g of THCA in the extract. And if we know how many grams of acid are in a given sample, and we know the volume of the sample, and we know the volume of the overall batch that the sample came from, then we can figure out exactly how many grams we have sitting in that extract.
The method I detail below is certainly not the way we did it in the labs at school, those are done with extreme precision and accuracy that’s too time consuming, and not really necessary here. This is basically a quick and dirty method that can be done in a minute or two, requires minimal product or equipment, and should still be accurate to a percentage point or so, which is more than enough.
What we did is stripped it all down to only the most essential information. That is:
- The volume of the unknown extract
- The weight (in grams) of the total NaOH used to react the acid with.
And that’s really it. So based on those simple needs, here’s what we came up with.
Things you need:
-
Sodium hydroxide (must be as close to 100% as you can get)
-
Pipette, or other way to accurately measure volumes in the 0.5-1 ml range, the more precise the better.
-
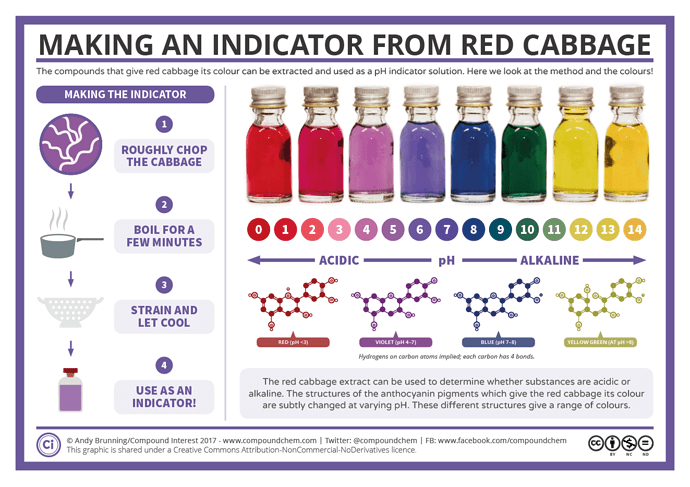
Indicator solution. There’s tons of fancy expensive reagents you can use if you have em, but they’re not necessary. Im using the phenol red that I picked up at the local Wal Mart for $5, it’s used to test PH in pools. But in a pinch, go buy a head of red cabbage at the grocery store, chop it up and boil it for 20 minutes. Strain the cabbage out and the thick syrup that’s left works just as good as anything else.
and at least one (preferably both) of:
- Eye dropper of some sort (the more precise the better)
- Small scale accurate down to at least 2 decimal places ($10 at your local smoke shop)
-
Carefully take out 10 ml your extract sample and place it in a small beaker. Be very precise with this measurement. Add another 10-20 ml or so of water, no need to be as precise with this measurement (it’s fine if the extract is in an immiscible solvent, we’re going to evaporate it off anyways, titrations need to be done in water only). Then place the beaker under a fan or somewhere it can evaporate (with minimal heat, there isn’t much acid in your 10 ml sample and you don’t want any to decarb, that would defeat the entire purpose of titrating it)
-
While waiting for the extract sample to evaporate, create your NaOH solution. If you understand the theory behind titration you can create any concentration of solution you wish, but for the system I came up with, you’ll need to create a 1.509 M solution of NaOH (I explain molarity in more detail at the bottom, just didn’t want to clog up the main post here). This can be done by adding 0.651 g NaOH in 10 ml of distilled water, or 6.35 g/NaOH in 100 ml.
-
Once that’s done, check on your extract sample. Once you’re sure all the solvent is gone, add a few drops of your indicator solution. Now simply slowly start adding NaOH solution to the extract, being very careful to record exactly how much you’re adding. You’ll get a sense for when you can go a bit quicker, and when you really need to slow down and be precise after a few titrations, but the measurements are such that for most extracts (which should be at or near saturation), you probably won’t need to worry about REALLY taking your time and being careful until at least 2-3 ml in. The reason for the weirdly specific concentration of 1.509 M NaOH is that the system is configured such that if you start with a 10 ml extract sample and if you titrate with a 1.509 M solution, then for every 1 ml of NaOH solution you drop in without reaching your equivalence point (i.e. the point the indicator changes color to the color of the equivalence point), your extract concentration goes up 5 g/L. THCA solubility in ethanol is 35 g/L, don’t know methanols but it would be higher. My point is, most extracts should have no problem clearing at least the 5 g/L bar, unless you used a lot of solvent and/or really shitty materiel. If your extract solution makes it past the first ml addition, then you know your extract is at least 5 g cannabinoic acids. If you get 2 ml with no change, you’re at 10 g/L etc etc.
Keep adding NaOH slowly until you get the color that indicates your equivalence point. That will depend on the indicator used, but one thing to note, you are NOT looking for PH 7. Equivalence point and PH neutral are not the same thing when reacting a weak acid with a strong base. the equivalence point of the example titration we did above would be at 7 PH, b/c that was a strong acid/strong base. Carboxylic acids are weak. If you don’t have any indicators you can use a PH meter, but it won’t be as accurate because they generally are not nearly accurate enough to detect the minute changes that indicators will (if using a PH meter, shoot for around an 8.5 ph). If you use the red cabbage indicator shown below, you can to get right in the spot between purple and blue. If you start to hit green, it’s too far
The reason I like phenol red is it doesn’t have gradations like the cabbage indicator does, and you don’t need to try to figure out if a certain shade is more blue, or more purple, or more green etc. it’s changes color only at the equivalence point, so it’s much less of a fuzzy area than the cabbage indicator is.
(the picture is mislabeled. phenol red doesn’t change color based on PH, it changes color at neutrality. It can seem like it does, b/c In water, or most aqueous solutions, or during a strong acid/strong base titration, neutrality IS at 7, but in our specific case, it isn’t. The reason is that with carboxylic acids, the salt created by neutralization is itself acidic and forms a buffer solution, and the neutrality point is at a higher PH)
You don’t want to overshoot, try to get it JUST as it changes at the equivalence point. Since we’re titrating very small amounts of a much larger batch, the calculations in the titration are then extrapolated, which means small mistakes can be multiplied and become inaccurate. Once that’s done calculate how many ml of your NaOH solution you used, and you can figure out your acid content in g/L. Multiply that number by how many L of extract you have (i.e. if you have 430 ml, mulitply by 0.430) and Bob’s your uncle.
Also, feel free to check the math on that titrant system. I’m about 95% sure it’s correct, but the math gets real wonky, and it could be off by a little bit.
Enjoy.
(The ‘M’ stands for “molarity” and all molarity is is a standardized unit of concentration, telling us how many moles/solute are in 1 L of solution. a 1 M solution of NaOH means that there’s 39.997 g in 1 L of solution. Since it’s a concentration number, we can use this benchmark to discuss any volume. So a 100 ml solution of 1 M concentration is a 100 ml solution of 3.997 mols of NaOH. a 2 L solution of 1 M is a 2 L solution that contains 79.994 mols/NaOH. Even though the volumes of all three solutions were very different, the concentration (1 M) of each one was the exact same.)