I had a heated debate at work today. We both agree pulling vacuum on your CLS before running is a best practice. I claim the reason you pull vacuum on your CLS before operating is to avoid possible combustion from a spark. That’s also why I bleed my nitrogen line before charging my tank. My lab manager claims the reason to pull vac is because we run an active system and oxygen is bad for the pump. This seemed kinda silly to me, but my explanation seemed silly to her. What is your input?
Oxygen is necessary for combustion, so for that it’s a safety precautions
Atmosphere has moisture in it which is the enemy of wax and can seize your lines with ice crystals if they’re small enough, so removing atmosphere is key to having clean, moisture free butane (in addition to changing sieves)
You cannot (under these conditions) compress atmosphere into a liquid, so it will contribute to head pressure/excess pressure.
Vacuums help pull solvent forward, as opposed to having a bunch of gas infront of the path to fight
Many more reasons I’m probably forgetting.
I know of the other benefits. Just didn’t want to write a book lol. She told me Butane can combust without oxygen (SILLY). I don’t believe oxygen would be bad for the pump considering it’s designed to handle straight propane.
shes wrong but just agree with her because you both already agree no oxygen allowed. If you like arguing with her tell her its the hydrogen that explodes if the pump touches it.
I mean just show her the definition of combustion. You cannot have combustion without oxygen.
Yep. Both those statements (combustion sans O2 & O2 damaging the pump) are complete and utter horse shit.
Telling the boss they’re wrong is not an art form I’ve perfected, but I really appreciate the ones that respect it when I do
I think she was having an off day and brain farted catastrophically. She’s a genius and her saying this threw me back. That’s why I had to take a step back and question what I thought was common knowledge.
…and give her references to the concept UPPER explosive limit. See: Flammability limit - Wikipedia

What boss, you didn’t know you can have TOO MUCH butane to explode?
Now you fucking do… (and it’s only 8%…)
Then there’s RTFM.
Check the pump manual, or call the support line. Testing function probably involves opening the inlet to atmosphere and shutting and off the outlet (high pressure shutoff test). Possibly pressurizing an external vessel the same way (CMEP-OL & Vaporsmarts both use this test).
The conversation started because we got a diamond miner. Never used one before always done jar tek & Pyrex tek. I asked if it was necessary to evacuate oxygen to prevent it being a spark hazard and oxidation. Is it necessary to evacuate atmosphere in a diamond miner?
You are right. Your manager isn’t wrong.
Yes. Or at least standard practice. Given that the UEL is 8%, logic suggests you could skip it without introducing a whole lot of risk. It’s certainly easier to perform closed transfers if you evacuate your target container.
I thought the valve on the diamond miner had to be open in order to get a good fill. That’s why I was confused on how to evacuate O2
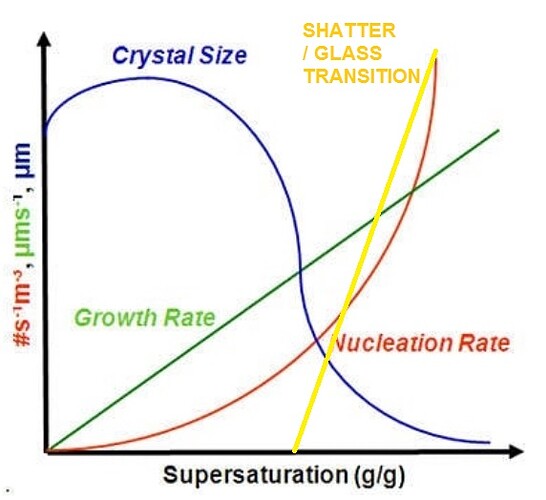
My understanding of it is that pressure slows down evaporation of your solvent, resulting in bigger rocks. Am I incorrect in thinking that? Which is how I explain why pentane results in nice diamond formation under less pressure.
it does if you don’t evacuate it first…or perhaps chase it in there with N2.
yeah, pressure is about keeping your solvent liquid so your cannabinoids can move around and find each other.
I’ve been approaching diamonds as more of a craft or art than a science and feeling it out to get results. This really puts things into perspective. Thank you!
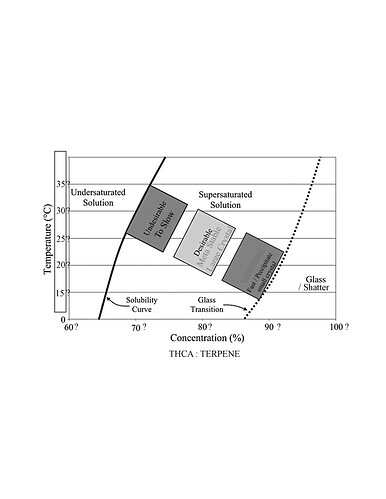
Do you know what solvent this chart is based on?
That I do not.
I’d assume butane.
But I’m probably wrong ![]()