Has anybody built or set up a pilot scale distillation apparatus we can use to break azeotrope with? We use these techniques sometimes in refineries petrochemical or for plastics.
Pressure swing or differential pressure and increased pressure distillation for breaking azeotropes?
Yes, it’s used with heat scaling.
have you seen it more specifically used for reproofing etoh, hexane, pentane or other solvents? is there any equipment or a apparatuses in production on this scale out there now?
Then process you are talking about that’s used in petrol uses hot steam to carry the volatiles further up the pipe that would normally be left behind. It is a version of sweeping. The other heavy azeotropes release from the vapor stream and fall down.
The solution is sweep gas and apollo. It will let you move molecules easier that would normaly come out in groups.
The other solution is scaling temps up and down as you are working. It creates pressure pulsing where vapor bubbles develop in the flask and push other molecules down, when scaling it prevents others from being pushed down. If that makes sense.
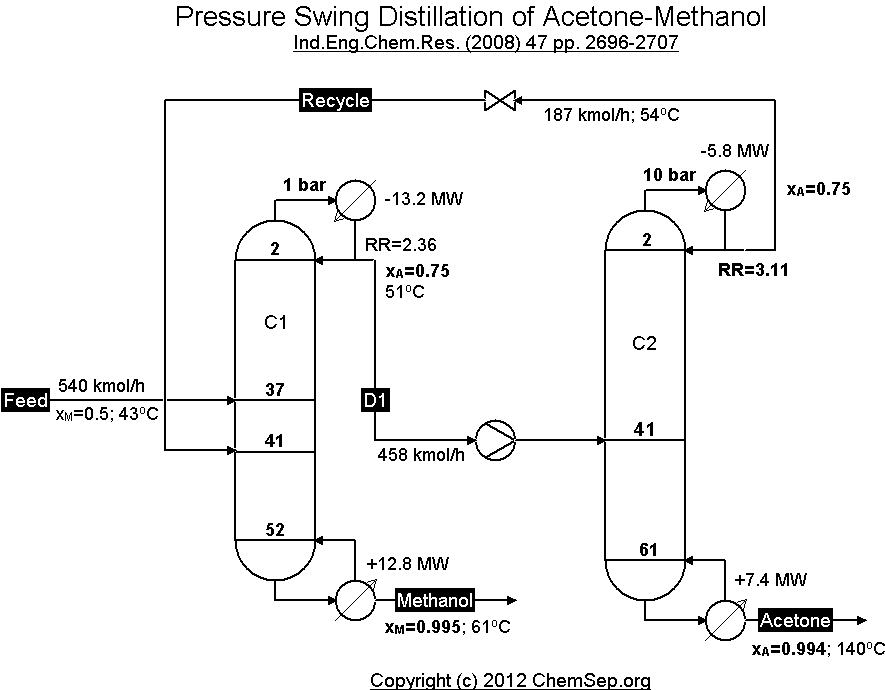
It makes sense buddy. That approach does seem to be in a sense a variance on what I’m mentioning but more precisely we use 2 separate distillation towers. The feedstock is introduced into tower 1 at x pressure. With a recycle loop between the 2 towers and tower 2 is at a different pressure. Perfect example is a methanol-acetone separation on a chemical plant.
would be interested to see a diy distillation spool capable of separating say hexane from etoh or other denaturing agents…
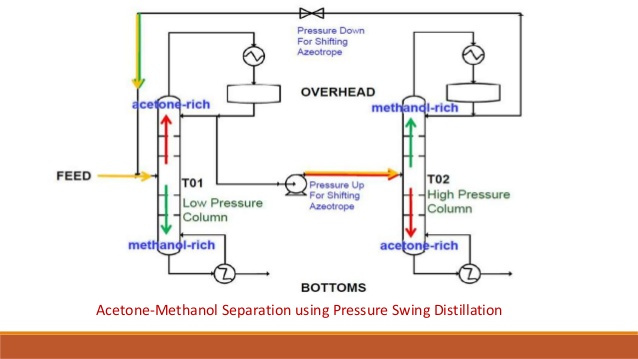
Pressure-swing distillation[edit]
Another method, pressure-swing distillation, relies on the fact that an azeotrope is pressure dependent. An azeotrope is not a range of concentrations that cannot be distilled, but the point at which the activity coefficients of the distillates are crossing one another. If the azeotrope can be “jumped over”, distillation can continue, although because the activity coefficients have crossed, the water will boil out of the remaining ethanol, rather than the ethanol out of the water as at lower concentrations.
To “jump” the azeotrope, the azeotrope can be moved by altering the pressure. Typically, pressure will be set such that the azeotrope will differ from the azeotrope at ambient pressure by some percent in either direction. For an ethanol-water mixture, that may be at 93.9% for 20bar overpressure, instead of 95.3% at ambient pressure. The distillation then works in the opposite direction, with the ethanol emerging in the bottoms and the water in the distillate. While in the low pressure column, ethanol is enriched on the way to the top end of the column, the high pressure column enriches ethanol on the bottom end, as ethanol is now the highboiler. The top product (water as distillate) is then again fed to the low pressure column, where the normal distillation is done. The bottom product of the low pressure column primarily consists of water, while the bottom stream of the high pressure column is nearly pure ethanol at concentrations of 99% or higher. Pressure swing distillation essentially inverts the K-values and subsequently inverts which end of the column each component comes out when compared to standard low pressure distillation.
Overall the pressure-swing distillation is a very robust and not so highly sophisticated method compared to multi component distillation or membrane processes, but the energy demand is in general higher. Also the investment cost of the distillation columns is higher, due to the pressure inside the vessels.