Hi everybody, I’m going to be trying some new things soon using hexane and pentane and I don’t have a fume Hood. I’d like to get myself a proper respirator to protect against these solvents specifically and more preferably. I did a search and did not find anybody talking about it here. I hope y’all are staying safe. What cartridgs/specs do we need to stay safe in this business?
Adequate and appropriate ventilation, while not technically PPE cannot be over-stressed. A fume hood it really the right place to play with pentane much above 10C imo.
Some flammable gas detectors can be worn, but more usually they are situated near the suspected source, so again not really PPE, but vital for worker safety.
As far as actual PPE goes, the standards of lab coat, close toed shoes, and safety glasses are a decent start.
Adding to that pretty much any “organic vapor” cartridge that has a NIOSH stamp should suffice.
Eyewash and shower in/right at the door of any room where things might go sideways is good practice.
And if that sounds like too much work, for anyone following along, at least play outside, have a garden hose you can find your way to with your eyes closed…and a buddy to help you find it or turn it on you.
There is no canister type filter that can filter out pentane and hexane. They are both extremely dangerous and can go through all mask type filters. I personally almost went blind from being exposed to pentane. It’s over a year later and my eyes are about 80% back to where they were. Osha says the only safe way is a supplied air system. I agree with osha. Dont learn the lesson the hard way!!
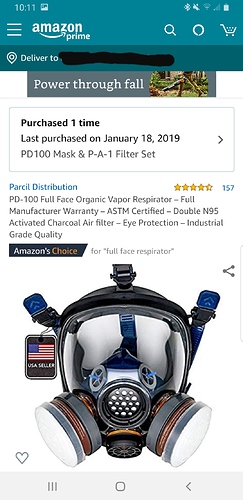
I use these with hexane/heptane and I can tell they work.
It was impossible to be inside soaking and not get high off the hexane without this mask tbh lol
Air flow does help though
Thanks everybody, I’m making crystals, or trying to for the first time. I’m planning on dissolving in the roto to catch the vapors when it’s warm and bringing the solvent to its saturation point at room temperature before jarring. I’m not sure it’ll pour out the flask when saturat d though, wondering if I need some kind of small condenser apparatus on my crystallization dish to contain those vapors if it needs to evaporate more.
I’m sure, I’ve been soaking in pure IPA under a window with a fan and even that stuff gets me, I’m a little scared of the hydrocarbons, I may even wear a snorkel with a tube running outside for fresh air. I’m pretty much a one man operation, so not too concerned with OSHA, I don’t trust their advice anyway. They allowed me to get chlorine poisoned at the food safety lab I used to work at.
As long as you have sufficient air flow and vapor cant build up you’re good.
If you had enough air flow you wouldnt even need a mask, then the problem is containing the smell of hydro carbons lol