There are many analytical techniques here but there is no reason to overthink this. I believe melting point will be your quickest most reliable data point to know if it is 97%+ CBN and compare to other reported melting points. If you do not have an apparatus you may be able to send some sample to me and I will get you melting point data, maybe even NMR data if you play your cards right
The sales rep that I’m dealing with told me “CBN in its purest form is pink” ![]() they are starting to slowly lose my trust
they are starting to slowly lose my trust
pH problems maybe?
I’m getting in touch with someone actually in the lab, as opposed to the sales rep, should have a straight answer soon
Just impurities.
Few ppm of a red colored pigment already have a strong impact on a white (uncolored) matrix.
@shattertramp does is smell anything special (besides a possibly slight heptane like odor) ?
Does it gets pinker if you expose it to light for a while ?
No smell, no taste, it’s already really pink unsure if light would affect, like long term exposure to light?
Mixed it in a tincture at 50mg/ml seems to work very well
Tell me you didn’t pay $12,000/kilo.
Waiting on more info from the lab, why? Do you think it’s not worth 12k, feel free to make a suggestion for a better product from another lab, advice is always welcome
No smell no taste is a good sign. You could just expose to sunlight a dilute tincture (in a transparent colorless solvent such as ethanol or MCT oil) to see if it gets pinker and pinker upon days of exposure (will take longer in MCT oil). Would give more hints about the stability of this product.
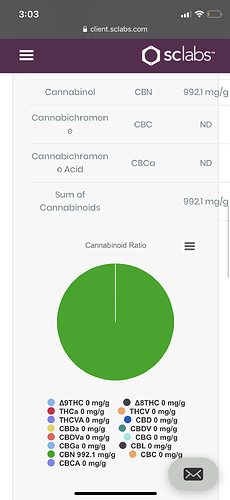
So. Here’s the response from the lab. Doesn’t seem like a straight answer.
Thoughts?
"Organic chemicals in their pure form absorb light in all the colors of the rainbow. The particular color of a chemical depends on its structure, the solvent in which it may be dissolved, the pH of the solution, the oxidation state of its surroundings, the environmental temperature, and many other factors.
In the special case of cannabinoids, they are known to appear in various colors. For example, pure CBDV undergoes dramatic color changes depending on the particular solvent in which it is dissolved. CBN has two aromatic rings and is a strong chromophore, appearing pinkish brown when a solid and changing colors when dissolved in various solvents.
The mechanism by which pure cannabinoids come in different colors is likely a consequence of a transient resonance phenomenon of the olivetol phenol such that there is a certain minute fraction of time in which the aromatic structure shifts to a quinone, which would have a particularly strong chromophoric signature and color. If this hypothesis is correct, there is no such thing as a stable “pure” cannabinoid per se, but rather the existence of a mixture of resonance forms whereby the aromatic and quinone structures are in a dynamic equilibrium almost entirely dominated by the phenol. To test this hypothesis one would need to run a specialized NMR for several weeks in order to detect and characterize the rare appearance of the putative quinone carbonyl. The special conditions of each cannabinoid (e.g. cyclization, length of chain attached to the phenol, type of terpene dementhane structure, etc) would influence the chromophore intensity at various wavelengths, thus imparting unique colors to each cannabinoid under various conditions."
Strongly considering this product still even with the pinkish coloring, any more input would be greatly appreciated thank you all for the responses
Sounds like there’s a Walter white amongst the CBN
It generally makes sense. But to me it is not the color of the cannabinoid, it is the color of the “isolate”, which include the main cannabinoid matrix, plus little others.
Can you send me a sample ?
I have a very clean method for isolates.
I can t at least tell you the level of impurity. And it would make a very good standard for me. Would ideally need at least a gram to be consistent (it needs about 100 mg/test, with 1 qualitative test and two replicate quantitative tests).
That pink colour may just ne due to light exposure at the end of the purification process. One recrystalization step carefully lead may remove this.
Theres a post around here somewhere where @Beaker (RIP my friend) was talking about how cbn refracts red light which makes it appear slightly red
Good ol’ WC15 is making CBN that is fully white, like, sparkling white micro-crystalline.
So perhaps it is impurities.
If anyone doing conversions wants to get rid of all mystery peaks and unknowns, hit me up. Our chromatography resin is excellent for removing conversion byproducts, we have a new polymer specifically designed for removal of sulfur compounds from CBN conversions, and other polymers for other byproducts as well. We have two PhD chemists on staff who have been developing polymers each for over 25 years.
Pink and purple d8 are common in my seeing… I imagine color remediation in distillation isn’t all Equal.
We’ve made 99% pink CBN, for us it typically happens after Chromatography when evaporating our water layer. The whole thing turns pink like a strawberry milk when there is a small amount of organic solvent and a lot of water with CBN ![]() Go figure.
Go figure.
Small ppm impurities with large extinction coefficients (lots of light absorbing ability) can cause color like this in materials. This is famous in ionic liquids, they’re always amber due to these ppm level impurities in the amines. Similarly to if you take a vat of water and put a drop of food coloring in it, you’ll be able to see the color in it.
I bet if you recrystallize this really slowly you could get the impurities out.