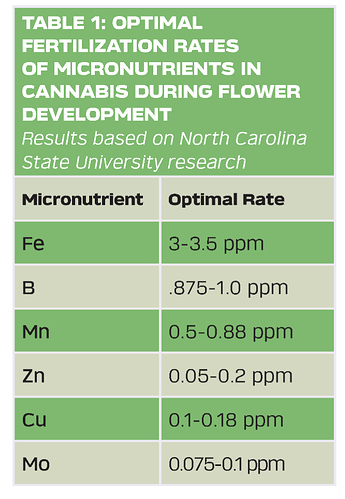
Hello everyone, I know I’ve asked this before but can anyone tell me some optimal elemental ppm ranges for micros? Can’t seem to find much on the topic. Any help is greatly appreciated.
Bump.
Thanks.
By what I’ve seen the cu and zn are way too low on them ratios
I see Harley Smith’s recommendations for Cu, and Zn are 0.1-.5, and 0.5-1 elemental ppm respectively.
This was the baseline I started at just figured I would share.
It is important to mention that some of the optimal micronutrient levels are very dependent on the concentration of other nutrients, the media, the pH or the actual form of the nutrient.
Here are some examples of how these things change micronutrient demand:
-
Requirements for Fe as Fe-EDDHA will be lower, as it is more highly available than Fe-DTPA or Fe-EDTA.
-
Coco will fix Cu and Mn, so higher levels of these nutrients are often required in coco to achieve the same composition in leaves.
-
High levels of P in solution will antagonize Zn and Cu, so higher levels of these nutrients are needed if higher levels of P are used (>50 ppm of P).
-
High VPD will lead to very high B transport to tissue, so much lower levels of B are required if the VPD is increased during flower.
-
Higher media pH will drastically lower Fe and Mn uptake, especially if using Fe-EDTA, Fe-DTPA or weaker chelates like Fe-citrate.
Researchers will usually grow plants under lower P and lower VPD than commercial cannabis growers generally target. It is also worth noting that North Carolina researchers only grow high CBD flower, not high THC flower.
Thanks Doc, a wealth of knowledge as usual.
How much more absorbable is the fe-eddha at higher ph than fe-edta? We’ve got issue’s with high ph in our rockwool plants in veg and the first 2 weeks of flower, I know it’s been affecting iron absorption.
Is that for hydro starting with 0 ppm water? i use soilless mixes so it must change alot depending on the medium. The house im in has a well and uses water softening but also has a water cleaning reverse osmosis. Bu a contracter sabotaged it? And its cut in a few places and when they brought in another guy he wanted nowhere near a sabotaged system.
It will fix your Fe uptake issues if they are related with pH. Replacing half of your total Fe with EDDHA should fix the problem. At pH 7 Fe-EDTA dissociates because Fe is falling out as Fe phosphates, so the uptake from Fe-EDTA starts to approach zero as it is removed from the solution.
High PH is effecting your iron absorption? I always errored on a higher PH side as cannabis tends to absorb iron no matter what.
Is there any disadvantages of running this at normal ph ranges 5.8-6.2. I got some ordered to try out when we have high ph issues.
@vortal yes higher ph can cause iron issues. I’ve been foliar feeding as a bandaid. Using agt-50 in my foliar for sure helps.
In my experience this depends on your Fe form, I routinely see low Fe in tissue with high pH in media, especially if you’re going above 7 and your forms of Fe are EDTA or DTPA. This is also common across many plants, as the chemical availability of Fe is just pH dependent.
Regardless of how good a plant is at Fe uptake, it will be very hard for plants to get Fe out once it precipitates out of solution. Luckily cannabis is not as bad as other plants in the same family, like strawberries!
@danielfp. Hi Doc, I was wondering if you happened across this article but any chance; and what’s your take on it?
Nirit’s research is very carefully done and her findings are conclusive for her growing conditions and cultivars.
The quality vs yield result is a classic observation among many plants, an effect called yield dilution. When you have higher yielding plants, you will often have lower secondary metabolites, which means the products are going to be lower quality. Obtaining the best compromise between the two has been a focus of plant research for the past 50 years.
It is therefore not entirely surprising that under nutrient deficient regimes the plant tends to yield dramatically less and therefore have better quality in whatever product is indeed produced.
It is interesting that she observes the opposite as it relates to Mg, with quality only decreasing as you decrease Mg below sufficiency and the optimal levels of Mg giving optimal quality too. In this case Mg should just be sufficient, really high Mg doesn’t cause problems under Nirit’s conditions but it will lead to bleaching under high intensity LED lights.
I have discussed the issue of K with Nirit personally a few times. We agree that nutritionally K doesn’t have any reason to be higher than 100ppm but in practice I often observe the best results in flower with much higher levels of K. This might have to do with issues related with accumulation of nutrients in media, but this is a much more extensive discussion.
However it seems clear to me that higher K’s results have nothing to do with higher K uptake, as this is not what I observe when K is fed higher (no higher K in flower or leaf tissue).
When looking at Nirit’s conclusions, bear in mind she grows under no CO2 enrichment and under pure perlite, with the exact same irrigation conditions through the entire crop (no adjustments of pH, EC, etc). Maintaining a 20% runoff.
Most growers won’t be under this type of regime. The CO2 enrichment will be high, the light intensity will be higher and there will often be a lot of changes done to both nutrient strength and irrigation practice through the crop.
I have never done a run with Nirit’s exact K recommendations under these different conditions, for other nutrients I fundamentally can agree with most of her findings and their extrapolation to other growing conditions.
Thanks Doc, I appreciate your feedback.
Tried swapping out half my Fe until the end of stretch when I can usually get my ph back in proper range of 6-6.2.
My mix is now really red and the stock tank looks like there’s blood in it lol.
It is VERY red indeed! This is a normal and often not awesome property of Fe-EDDHA.