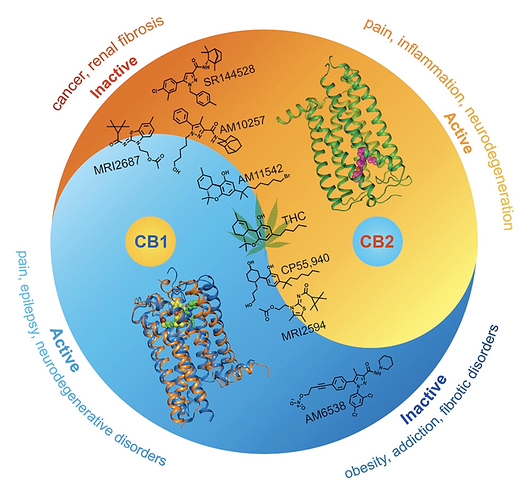
Yes, if you tried delta-9 and other stronger CB1 agonists, it’s very apparent it’s activity is due it is CB1 activity.
It binds weakly to other receptors, including opioid receptors and the transient ion channels(hot/cold thermoreceptors) but its apparent that when taken alone that CB1 activity is d9THCs major effect. Other receptor antagonists only weakly reduce THCs psychoactive effect proving this but need to dig up those studies
Antagonists are the best way to show what receptor is causing a targeted effect. If a highly selective receptor antagonist blocks the targeted effect but not others, you can prove that receptors agonists is there cause of that targeted effect.(Note:CB1 antagonists in high doses, especially irreversible antagonist have caused death in humans, don’t fuck around with these)
Basically many have only been focused on the chemistry of making these compounds , when pharmacology and pharmacokinetics are the much more important concern to prove safety and efficacy
6 Likes
Does this antagonist O-1057 function similarly to SR141716A and SR144528? I know the FDA didn’t approve its use in the US because of its GABA inhibiting action and I believe with another neurotransmitter/receptor.
It’s only used in research from my understanding, not meant for use in humans. Think it was another one of that series, that one is water soluble which I think is why I remembered it.
Yes was O-1258 lol, it’s very similar to rimonabant so unlikely to be approved
I have read that several antagonists that were being researched or developed after the FDAs decision were scrapped. I haven’t seen tons of research on CB1 antagonists past that point, which I believe took place in the 90s, I’d love to see some if there is.
Could CB1 antagonist fatalities be related to the theories on the endocannabinoid system’s relationship to homeostasis?
Also I was under the impression 10a6a is also only used in research, mainly back when they were studying the cannabinoid receptors and didn’t have access to isolated D9
1 Like
It’s analogue Parahexyl was tested in humans
1 Like
I found a journal that may contain info on parahexyl, CB1 receptor antagonists: new discoveries leading to new perspectives, have you read this one before? I’ll need to find a way to access it other than this site, https://onlinelibrary.wiley.com/doi/full/10.1111/j.1748-1716.2011.02402.x
Edit: journal is easily accessed on sci-hub
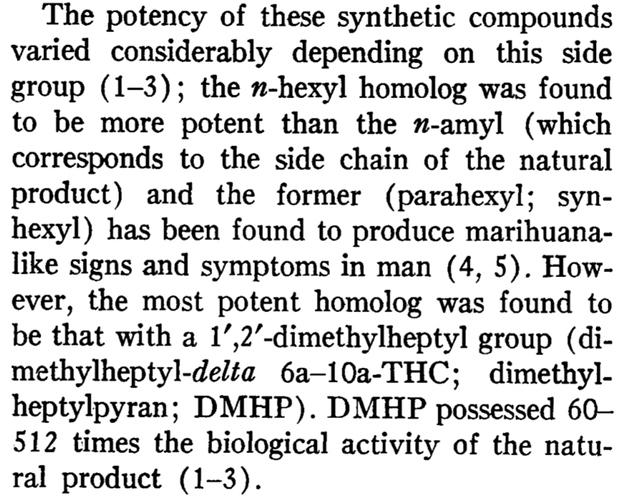
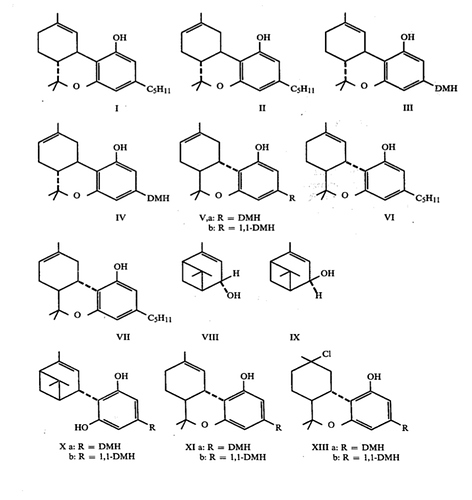
Yep and it’s action is directly tied to its CB1 affinity. This was proven through the mentioned analogue parahexyl. Parahexyl only differs by its hexyl tail. This modification increases potency by increasing affinity at CB1. The HU- synthetic cannabinoid series utilizes a dimethylheptyl tail and increases potency 1-800x(HU-210, is also more potent to 11-hydroxy addition as well which we see in regular THC). The sites of these modifications are called pharmacophores, sites that directly effects the receptor affinity of a molecule. The tail at 3 position and the 11 position are examples of this. There are 4 known on THC and their Structure-Activity Relationships(SAR) have been studied for decades. Another pharmacophore is the double bond placement which has been the focus of interest here.
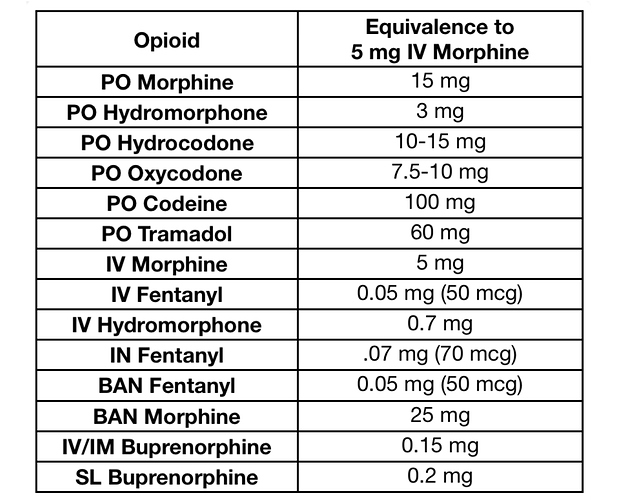
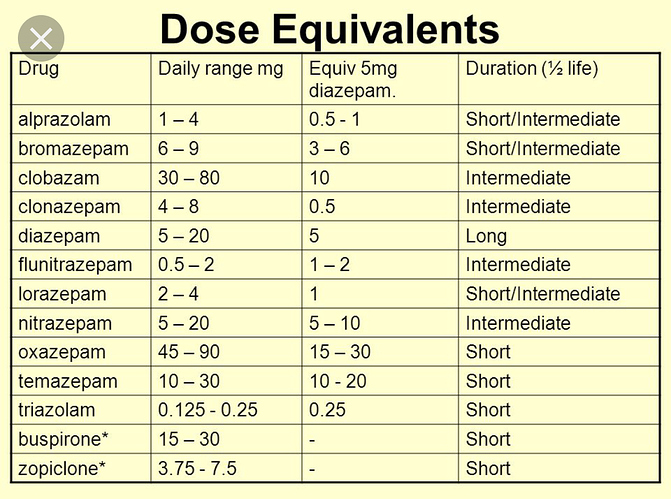
Older and more utilized substances have dose comparison charts; as substances that act on the same receptor generally feel very similar, just differ in dosage and duration.
Opioid dosages are all based on Morphine potency, Psychedelics traditionally were based on Mescaline potency
If you check affinities for mu receptor for opioids and GABA receptor for Benzos, you’ll find a direct correlation to affinity and dosage, though things like metabolic conversion rates to other more/less active compounds effect potency as well as some other factors.
Hopefully one day we can have something similar for cannabinoid medicines
(Edit to previous post, the event that was originally attributed to the CB1 irreversible antagonist effect of a certain drug appears is now attributed to another action(FAAH inhibition) of the experimental drug, substance was known as BIA 10-2474)
6 Likes
Are we off topic yet?
Cause this is #^<<€^!*ing awesome 
4 Likes
Dimethylheptyl-delta 6a-10a-tetrahydrocannabinol: effects after parenteral administration to man.
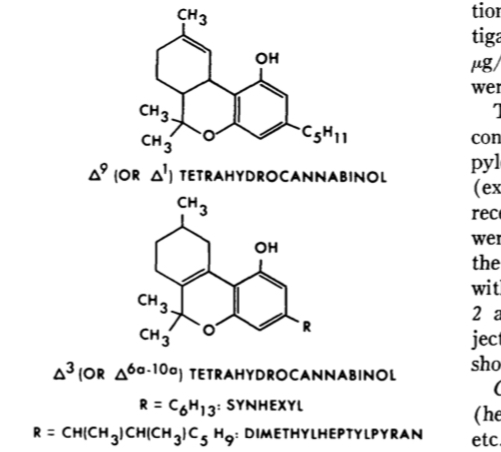
Modification comparison of 6a,10 with different tails, 1,2-dimethylheptyl being the most potent, as much as 516x more
1 Like
Awesome info dropping in this thread wow
1 Like
Point of all of that is, if we can make the same cannabinoids we know and love hundreds of times more potent, will current cannabinoids retain their value?
Most don’t know these substances exist but they have for a long time now. Why not utilize them? When one gram of a substances can treat hundreds, isn’t that more worthwhile then the very weak minor cannabinoids that exist?
Even if just used for research, wouldn’t more potent analogues be the better focus?
2 Likes
the Direct 6a10a dimethylheptyl analogues are already listed Schedule 1 Sundance’s unfortunately, though d9s are not.
Potency modifications are more interesting for substances like CBN or CBG. It is to be noted that the analogues mentioned are only made fully synthetically and are not made for naturally occurring cannabinoids due the the impracticality of cleaving and then adding carbon bonds at that site
1 Like
The big picture to me seems to be that of the many possible variations of cannabinoids, and other related constituents of cannabis, whether they’re biosynthsized phytocannabinoids, semi-synthetic, or endogenous their value on the market should be based on a combination of supply, demand, difficulty to produce, and efficacy, at least IMO. I wouldn’t make too many predictions on the value of those compounds before we see real developments in the economics of them. CBG and CBN being the two that right now are gaining the most traction, may offer more insight over the next few years.
Now, producing small samples of cannabinoids for research, that’s always welcome within reason.
Do you know which compounds are agonists antagonists of CB2?
Not as well as CB1 but lists are out there. CB2 structure was just elucidated with an agonist bound it, though, which is very interesting. 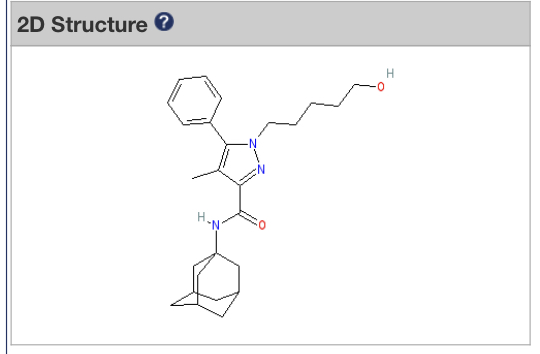
3 Likes