It should increase D9. Higher dilution and lower temps will favour D9.
Quite the contrary. Alcohols are great for use with Pd/C.
That’s incorrect. If you’ve ever filtered off hydrogen loaded Pd/C without an inert atmosphere, you’ll know it works at whatever fucking temperature it pleases to.
The question would be, do you want to hydrogenate or oxidise? With all the other components in there, they would react along with D9 either way. Some of them probably even faster than D9, so that would limit usefulness drastically.
Starts working on cannabinoids (d10)
Guess your a man of details
Make a mistake with pd/c on alkohols and you can make an interesting flame thrower
that has nothing to do with alcohols. any flammable solvent with Pd/C poses a fire hazard.
Please don’t spread misinformation.
Yes … there are several Lewis acids that thrive in water. My results are coming soon but for now that’s all I’m saying.
Beautiful
Didn’t know which thread to post this in but I wanted to share a mishap that I believe we have solved. Would love to hear others input as well.
We ran our typical isomerization SOP which has been very consistent/predictable until this run last week. The only difference is we used 900g isolate to 1300g Solvent as we didn’t realize we were short on isolate. We ran our rxn, but noticed we were not seeing significant exothermic heat production nor much color change. We ended up running it 30min longer than we usually do, proceeded with our washes, Solvent recovery, and distillation. Our distillate came out dark purple/pink. We assumed it was pH issues, and decided to rewash, then redistill. No dice, same color. I finally decided it was time to get some KOH and run an alkaline beam test. After a few tries of getting the hang of it, I believe I have nailed down the issue. I believe where we never had our exothermic spike, our RXN did not complete, and we still have significant CBD left in our solution. Here is a picture of our distillate, as well as a TLC plate of the beam test. Would someone more knowledgeable than I concur with my assessment?
@Miles-Beyond @HeisenbergInd, do yall have experience with alkaline beam test? I thought I read that you guys were using it to monitor your RXNs
Care of @cyclopath
Care of @Photon_noir
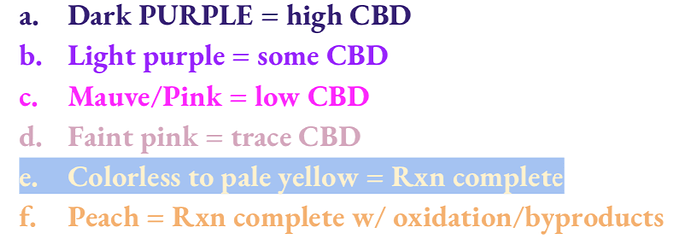
Alkaline Beam Test: Colorimetric testing for CBD
Preparation of Solution & Workspace
- Fill plastic graduated cylinder with about 70mL of 95% ethanol.
- Weigh out 4g KOH on wax paper and add it to alcohol.
- Swirl or stir with stainless or glass rod to dissolve all KOH.
- Finish filling graduated cylinder up to 100mL line with ethanol
- Label the clean glass jar “5% KOH in EtOH, CBD Beam Test” or similar, then pour the entire solutioninto the jar and seal the lid. Swirl a bit to mix well.
- On a paper towel next to the jar, write “Beam” or similar and place a new plastic pipette on it.
- On another paper towel next to the reactor, write “RXN” or similar and place a new plastic pipette onit, also.
- Set up several test tubes with lids in a rack next to the KOH solution and Beam pipette.
☆ Sampling & Testing
- Squirt the rxn solution sample into the uncapped test tube in the rack.
- Unseal the jar and remove the lid (or lid ring) to pull a little bit of KOH test soln from it. Only a fewdrops are needed.
- Squeeze one (1) drop test soln into the tube with the rxn sample and swirl the tube for 2 seconds tomix.
- Observe the color after 5 seconds. If color is light or invisible, add 1 to 3 more drops of KOH soln,swirl 2s. Odd color may show up upon resting for too long or excessive stirring, so only read color reactions that occur immediately (i.e. 1st color under 30 seconds).
- Take notes with time and dispose of sample.
I’d say you’re spot on with your findings. Having the extra heptane will “dilute” the acid and heptane solution. If you find yourself in that situation again, add more pTSA per volume of heptane to see if that works. That’s one thing I haven’t tried but in theory, should work.
Think of it like this…
Adding too much milk while making cook and serve pudding (butterscotch is my fav ![]() ). It won’t set up right. So, you half to crack open another box of pudding to even out the ratios. That’s why the 1:1 ratio of isolate to solvent has been a staple.
). It won’t set up right. So, you half to crack open another box of pudding to even out the ratios. That’s why the 1:1 ratio of isolate to solvent has been a staple.
No worries… chalk it up as knowledge and learn from it!
I appreciate your opinion on my beam test! I concur with your assesment. Tomorrow we are going to rereun the RXN and see if we are able to fix it. We already made a batch of water clear yesterday so we are not pressed for product. I’ve just been using this batch as a knowledge acquisition endeavor. I think it will be interesting to see after all we have done so far, in addition to re-running the RXN if we are able to remediate this batch and come out with water clear distillate. In theory, I don’t see why we wouldn’t be able to as byproducts should not be an issue. My only question is whether oxidation will affect the color. Do you know a procedure for remediating oxidized distillate?
A little birdie around the forum mentioned H2O2 in an LLE at 10% to volume of water.
ie: 900ml of distilled water and 100ml of H202.
Tried it before and it cleared it up a lot and didn’t hurt the end product.
Wise counsel @HeisenbergInd
That does work.
You are too cool man! Thanks for that, I cant wait to play with it! I’ve got 5gal of 30% H2O2 that I can use. So I might throw that into my wash routine for this next run depending on what it looks like at that point. I’ll post results here on what it looks like at various stages. Definitely gonna be interesting, to me at least!
Try to help where I can!
I’ve had enough free knowledge dropped on me, it’s only fair to pay it forward. ![]()
Isn’t this what Future 4200 is supposed to be used for?
Lately its been more like a menstruating laceration.
Glad to see knowledge continues.
Axe wound.
Thanks, @HeisenbergInd …although you are technically posting some of my confidential information. Tsk-tsk! ![]()
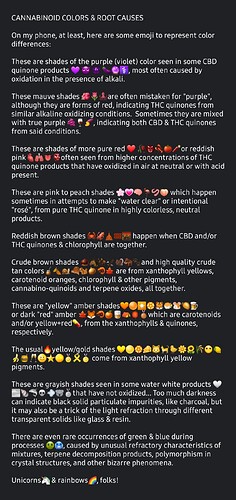
Here is the other recent post I made, but in screenshot form to keep the emoji colors accurate: