Throw your jar in here and it’ll be decarbed in no time! ![]()
The difference is “distillate” vs “isolate”.
So distillate is microwaveable and isolate isn’t ? Is there a simple explanation for this?
Hey bud, I get it. I was not asking for chain of custody, any paperwork, or legal ho-ha. I just asked if you made it or bought it,
I don’t care if its compliant, who grew it, etc. I wanted to make sure A) I wasn’t doing work for a competitor in our field. B) Some basic information to feed our analytical team in the event they have questions(and they will). If you don’t want to test your mystery oil, no sweat off my back. I thought you came here because you wanted share what your learned and learn something new in the process.
Déjà vu
It’s how microwaves work…
Crystalline solid vs liquid
If the molecules are unable to freely vibrate/rotate, they can’t convert microwaves to heat. Limited movement means there’s less kinetic energy to be converted to thermal energy.
When water freezes the molecules form hydrogen bonds. These hydrogen bonds keep the molecules from rotating freely.
If you watch the first video I posted, you’ll see that you can melt glass in a microwave. You just need a thermal boost to get the molecules moving.
You can heat THCa as you’ve demonstrated, just not as easy.
Lol microwave reactors are a thing i have one sitting in storage i never use because it was too powerful for the reactions i was testing at the time.
Ill throw yall a spoon though.
First off; decarb by its nature is a thermally driven unimolecular reaction. What that means is it requires a certain ammount of activation energy to surpass the bond dissociation barrier in order to cleave off the -cooh group.
In other words you have to make the molecule vibrate beyond a certain frequency to make it break the weakest bond of the carboxyl group
That specific bond disociation barrier is reported in literature at roughly 110-130 Kj/mol or 26-31 kcal/mol(i prefer to work in kcal/mol). this is a very low energy threshold in the grand scheme of things this also; just so you know, doesnt describe the heat required but the kinetic energy that is a side effect of latent heat input required to surpass the bond dissociation barrier.
We can run some numbers on this to prove that it can work for decarb.
Lets say we have a 1200 watt microwave. Pretty standard appliance that every house has.
1watt = 1joule/sec so a 1200 watt is theoretically capable of putting out 1200 joules a second or 1.2Kj/s.
We then convert that to k/cal per min
A 1200 watt microwave will give us a theoretical 17.2 kcal/min of potential energy (no system is perfect so it wont be this, consumer microwaves are noisy and ineffecient)
So in theory; its certainly possible you just need to sustain a temperature that lets a fraction of molecules reach that energy barrier. The system doesnt need to deliver a sustained 26-31 kcal/mol in net heat; the system just needs enough thermal energy distributed in the system for molecules to cross the barrier
So yes this is very possible. Microwave assisted synthesis is a rabbit hole i went deep into. This comment is a kind of gross oversimplification of something that is very nuanced, but hits all the major points without being a novel.
I thought that was the very clear point I was making.@ hempzotics: please do the ice cube demonstrations, then ask GROK “what’s up with that”….Then put the same weight of liquid water and do the same cycle…
I think it is pseudo unimolecular, with a proton transfer and keto enol transition, to a state where water or acid acts catalytically? A couple of papers on THCa and one on CBDA . You have a very strong intramolecular H bonded , 6 membered ring, in both the cannabinoic acid neutral form and the carboxylate anion form. You really have to know what you are heating up. If you have one of those microwave reactors, you probably have some of those capped containers , with teflon base and nylon screw tops that are nearly microwave transparent. so you can take a close look. Do’nt forget to drive moisture. Off with 30 sec low duty cycle pulses.
THCA white opaque crystal , when powdered and placed. On glassine weighing paper in a Labwave 9000, and pulsed at like 40% power rating…will rapidly loose what little water is there, and during this time it seems to change from white powder to a crunchy remelt that is off color a bit. But one you hit this dry microwave stage, you seem to be done with the material heating at all.
The Labwave 9000 has a little microwave transparent basket…you can put your glassine or bakers paper on.
Blast the paper one or two times right before using, some seem to have enough water ? To change form a bit.
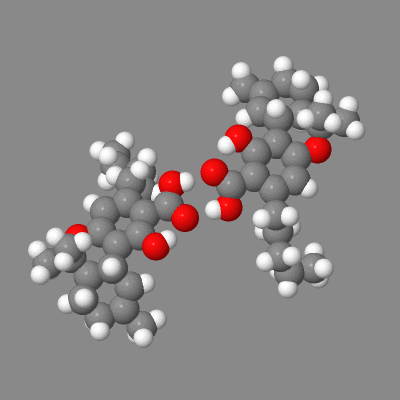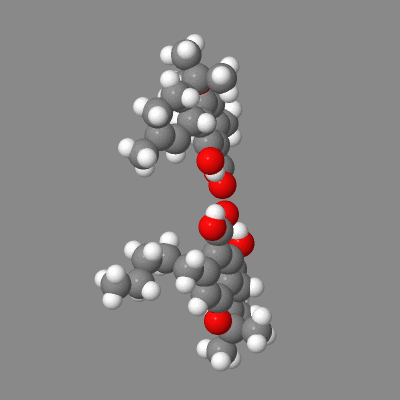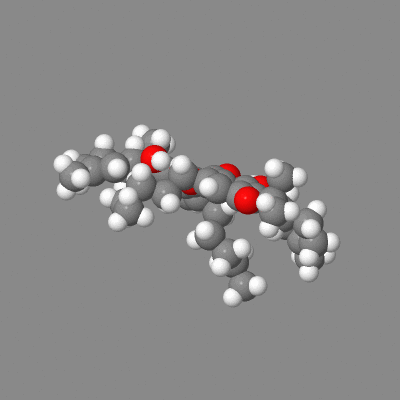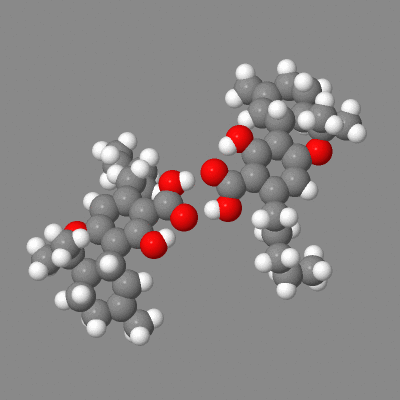
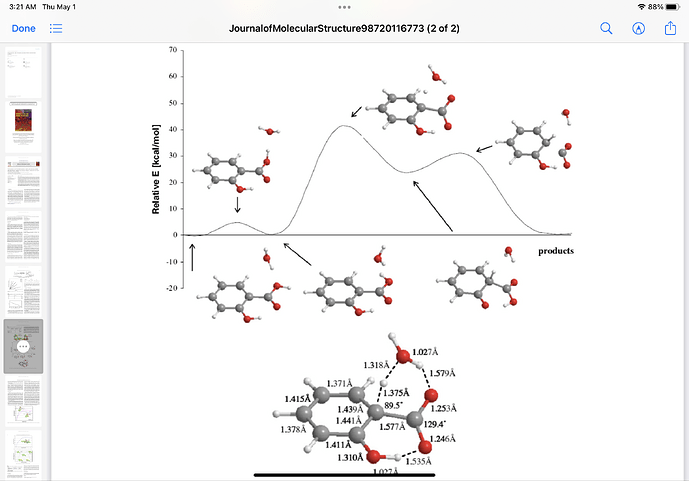
Here is a model you can play with showing both intermolecular h-bonding of dimers (some crystals) and the intramolecular-h-bonded , six membered ring.
Always appreciate your feedback!
I have a chinese made mcr-3 if i remember correctly, its a blue box with a cutout/relief pathway for a vapor duct and an integrated stir plate, the system came with 3 neck RBF’s and a bunch of other adapters and whatnot; for the price i paid($4500 for the unit with 2 extra sets of glass) i think they are just 3.3 borosilicate but i could be wrong.
I used the system mainly for looking at early cbd-d9 reactions and how i could speed them up, it was a pretty fun toy for a while, got me thinking about all sorts of things like microwave assisted distillation and whatnot. I ended up getting busy with other stuff and it went into storage.
But alas the techniques/methods developed in the system were not suited for realworld deployment due to the lack of true scalability.
I could indeed increase the reaction rate of cbd to d9 but lost some selectivity, atleast with the methods trialed /vs their batch synth in jacketed reactors.
I had relatively decent sucess with acidic cation exchange resins with the system, but again the lack of batch scalbility made the reduced reaction times sorta moot. I though about ways to make it viable in a production enviroment, but market conditions and more important projects took precident. Was a fun few months with that thing we named it “easy bake” ![]()
So if I had a microwave container that didn’t heat up on the microwave and put the diamonds in it, I would need to potentially add some water to the diamonds to create enough heat to decarb?
Could be down to try, and guess on how much water to Thca would be required?
Also what’s a good small container you suggest for doing this?
Microwaves are interesting, blew my mind when an extractor I was working with was microwaving rubbing alcohol in the microwave to clean with, I figured it would ignite it but it just heats it up and boils it. Always cleaned my rigs this way now, just some ribbing alcohol on it and pop in then microwave and it’s clean in seconds lol
Please don’t do this.
Yeah agreed.. lol the LELs of a tiny box will be exceeded very quickly… lots of ignition points. Really dumb.
My microwave reactor backfilled the entire system box with n2. It also gave a sealed pathway for vapors to travel and condense. i still blanketed the reactions seperately with a flushing adapter and bubbler setup. When i first started running it; it still felt unsafe cause of janky chinese engineering and chinglish warning stickers and HMI prompts.
Consumer microwaves should never ever be used with flamable solvents. Ever. Saving a few minutes isnt worth a fireball in your face.
Well I’ve done it a thousand times in cheap consumer microwaves with no issues. Fumes are prolly the worst of it. It goes against logic but in practice it does not ignite. Maybe if you let it run for 5-10 minutes straight. But 30-60 seconds it’s safe, from ignition standpoint.
Its not about exceeding autoignition temperatures, its about creating an explosive atmosphere and having some spark from the system or room ignite it.
Dangerous when it becomes superheated…
It’s the “fumes” that are the concern here.
Ignited by the high energy electronics in the microwave rather than via autoignition.
Do you understand LEL and UEL?
For iso you’re looking at
Lower explosive limit (LEL) 2.0%
Upper explosive limit (UEL) 12.7% at 200°F
You’ve done it a bunch,and gotten away with it…
Which tells us that actual “sparks” inside a consumer microwave are not all that common. Fair enough. They really shouldn’t be.
I still suggest caution. Wear appropriate ppe & assume every time might be the time it does blow. ie have an extinguisher near by.
Edit:
Fuck yeah, even water gets wild when it suddenly goes from liquid to gas. Water vapor takes up like 1600x the space liquid does at 100C…
I’ve had flasks erupt on me when touched on the way out of the microwave.
I wonder if that was a squeeze bottle and the vapors didnt have a pathway to esacpe thats what it sounds like to me.
The point is, you might have gotten away with it hundreds of times. You might even continue to do it with no problem for the rest of your life. It would be a disservice to you or anyone reading the thread to not immediately point out that safe=/=potentially hazardous.
Regardless, there are teflon containers made specifically for microwave digestion, I would look into those if you’re interested in still continuing down this line of inquiry about microwave decarbing.
Im not regularly doing it anymore. Just thought on the subject of microwaving things it was interesting and defied logic. Thanks for the safety tips tho guys!
I’m more curious about an air fryer for decarb.