I really appreciate all those photos and videos. Since your product is hemp derived and legal to ship, I’d be happy to perform full analytics(before and after) for free on your process and share all the data here on future. DM me if you want to work something out. Your THC-A looks extremely clean btw.
Send the addy
do you have any COAs of this microwave decarbed isolate?
Not yet
I mean ok ok ok ok.
Keep it simple stupid, eh?
I’d have always thought that there would need to be some liquid fraction to help speed things up, and I can’t explain why I thought that?
Brb bringing a microwave into the lab.
Decarbing cbd isolate?? What is the purpose of this?? Does it not just recrystallize?
I would decarb cbd isolate then incorporate cbt and terps for vape pods. But yeah if left to cool it would re-crystallize
Cbd isolate is already decarbd. Why would you need to decarb it again?
Decarbing CBD isolate?!?
Care to back that assertion up with COA’s on your input material?
Given that almost nobody is selling CBDa isolate, I would posit you’ve simply been melting CBD.
…because all you did was melt it…
Liters of “isolate”?!?
Much like THCa isolate, CBD isolate is a crystalline solid. Care to elaborate on why one would purchase that by the liter?
Sounds more like “crystallized CBD distillate”, which you then melted…
@moronnabis, what do you make of @Hempzotics nukeleation show and tell?
Yeah, like water. Or something else with bonds that absorb at 2.4GHz.
…but the 1k word substitutes above would seem to disagree.
Given CBD isolate is by definition sans carboxyl?
There is none…although it does melt the CBD, which can be useful if you’re working with crystallized distillate (rather than isolate)
I melted it I guess sorry for misphrasing, I was making vape pods with it.
I meant that I would microwave kilo worth of cbd isolate to melt down for carts, the sample I did with the Thca is only about 15g of micro diamonds. I haven’t attemptd doing a kilo of it at a time in the microwave but have MELTED cbd isolate in that volume years ago so figure it’s doable
I do think I see some remaining crystals in the sample I did yesterday so guessing I didn’t fully decarb it, it didn’t completely crash out tho I’m confident there was decarbing and not just melting occurring with that sample but without labs I can only guess. Will look to test at some point but in no hurry I just thought it was interesting tech for decarb and curious if any reactions or conversions could occur from doing it this way.
Maybe some of yall can do your own experiments and report back
It appears that @Hempzotics show and tell represents putting Crystalline THCA (but no COA) into unknown quality Chinese glass container and popping it into the microwave. Primary control would be: put the glass container empty into the microwave and ZAP it until it either breaks or turns very hot? I have stated that glass can absorb kitchen microwave radiation and heat up. Even glass that is “microwave safe” or microwave “rated” heats up from standard duty cycle with in minutes of repeated heating. The rating of the microwave safe glass, just means it will not break in the microwave, it does not mean it won’t heat up.
Non-scientific microwave ovens with the spinning glass plates will heat up with nothing in it. So Heating a glass jar with THCA crystals in the container, may well be expected to reach decarb temp. THIS DOES NOT MEAN THCA CRYSTALS ABSORB MICROWAVES. And as Such, the show and tell does “look like a decarb reaction”..
Usually we have found a bit of water associated with crystalline forms of THCA. It would seem that THCA crystals may have about a 1 mole h20 to 2 mole THCA ratio..about 2.5%. When assaying with a Labwave 9000 system. (When using 200 mgs or so of THCA, but may be close to the limit of detections.). And using THCA crystal purchased at local dispensary . So this small amount of water will rapidly heat and vaporize when first subjecting THCA to microwave. I recommend turning duty cycle down to 20% and doing repeated 30 sec zaps with a minute or so rest in between test cycles. Carefully done, the THCA may change form just a bit. Now mind you, this has to be done on something like baking paper, that has it self been preZapped to remove any water. Now if you have a Microwave “Transparent container that does not heat up when subject to microwave radiation” and an OVEN that does not heat up (powerful venting fans) ,..you can sort of have this discussion about microwave decarbing??? THCA, CBDA and CBD crystalline forms do not heat in the standard microwave kitchen oven appreciably by microwave absorption. I think the semi liquid state of THC may be different, but needs more study. But if you can heat the glass enough to melt these crystalline forms to liquid THCA, CBDA and CBD, those liquids may have enhanced microwave absorption. I do not know about liquid state.
@ganjineer710 has made a nice offer to the OP regarding analysis of the pre and post THCA show and tell. Let us get that done. He has advanced analytical support, and is very knowledgeable.
@cyclopath, So pop you glass container in the microwave, ZAP it , and see if you can pick it up by hand , if it heats up, there is a high probability that you can heat THCA, melt it and decarb with sufficient heating of the glass and enough time. The bubbling of the THCA in the show and tell makes me think he has heated it by heating the glass. And may be generating CO2…by decarb.
The rest about decarbing CBD is just a mistaken word salad by OP…I think he just melts it.
I’m betting those glass jars get super hot.
A good example here is try to heat cyrstalline water (ice) using microwave oven.
Lots of artists use microwaves to fuse glass. Kills the microwave, but they’ll take a fair amount of abuse.
Thought - One could simply add water, no? It goes poof at decarb temps anyway, so, hey?
If it takes decarb from several hours to like, 1 hr or less with a bit of water and some fiddling, how many microwaves would one have to kill in a month before the idea gets a lil wacky? How many microwaves equals the cost of a Cascade…errr…DDS?? vacuum oven anyway?
Obviously it wouldn’t quite make sense if you’re decarbing 10kilo batches of crude, but it’s a fun concept to play around with.
Imagine a microwave purpose built for this.
![]()
Thank you!!
Learned something: meansI can go home now…
Edit: I was aware of the microwave kilns as shown in the second video.
Very fun idea.
The only risks I would think of is overheating and oxidation byproducts.
I’ve thought about doing this in the past, but I don’t feel like shelling out the money for some THCa to test it with. I have had experience with other decarboxylations and it tends to follow the same procedure for each individual chemical.
From your original question, this should be a superior way to do it, but there are some ways to improve it. First, probably use some proper glassware rather than just jars. They tend to break under heat and are just unreliable. I would use some GL45 bottles, you can get a 1L one on amazon for about $15. Second, don’t close the lid during the decarboxylation. Besides pressure build up (very dangerous when working with glass), there is the slight chance that the reaction can reverse and you would not end up decarbing everything. This is minor compared to the pressure issue though. If you really wanted to scale up, I provided a good way below.
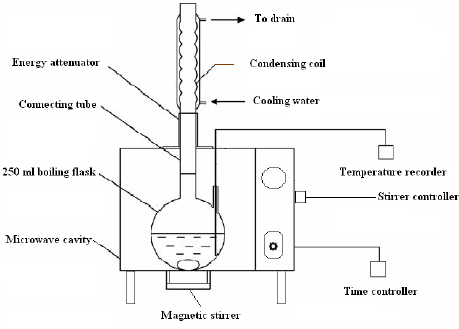
Microwave chemistry is long established and honestly a bit underused. I have seen a lot of papers cite its ability to directly and uniformly heat mixtures to be good for thermodynamicly controlled reactions such as decarboxylations. There is also a ton of papers on how to modify a commercially available microwave to enable this kind of reaction (see picture below).
For larger scale, one could do it in either a solvent or on its own. I don’t know how the two would compare, but I would use a solvent. Water should work well but it’s boiling point is slightly below the ideal (rate wise) temperature, but should give a near complete conversion. There’s a paper out there that goes over the decarboxylation rate of THCa at various temperatures and at 100C, its near complete after 60 minutes. This would take significantly less time in a microwave however as it was tested in an oven.
You would want to use a solvent to make sure that you don’t overheat your THCa and degrade it. Add a condenser to the top to return any water that boiled would be necessary. I would also vacuum and purge the apparatus to remove any oxygen that may be dissolved in the water, but you could get away with just boiling the water before hand to drive off any disolved gases. I would also run this under an inert atmosphere to minimize possible oxidation products. The inert atmosphere would be ideal, but the water should also provide a good vapor blanket while boiling.
A little note on how long to heat, a good rule of thumb is to just heat until bubbles stop forming. Decarboxylation have a very peculiar sound that is very similar to the sound of something frying. Generally, the decarb will be done when this sound stops.
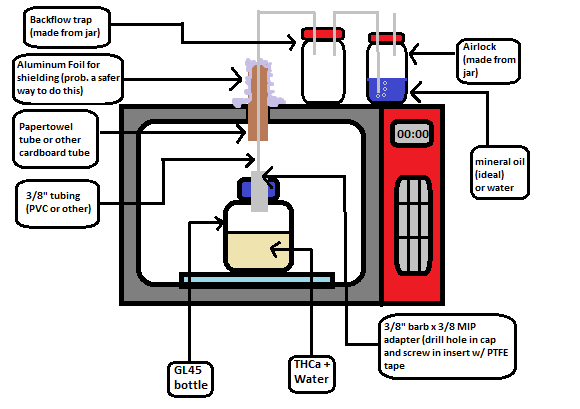
I’ve drawn up a little schematic for a cheaper version in case anyone don’t have any glassware and doesn’t want to drop a bunch of money on what may not work. One would have to add extra water in this since it would boil off during the decarb, but this could be solved with a condenser or air cooled pipe between the outlet and backflow trap. This could also run without water and the escaping CO2 would create an inert atmosphere, but you would have to keep an eye on the temperature.
Best of luck with this! If you can get it to work it would be quite economical.
So the glass is heating up in the microwave and causing melting/decarb, the Thca isn’t being heated by the microwave? I can’t think of why this wouldn’t be the case but why does microwave heat distillate so fast? Throw a media bottle in microwave and after a few 30-60 second bursts the distillate starts loosening through out. Seems like more is happening there than the glass heating up, perhaps not though.
@Ganjineer710 asking for chain of custody to test samples, I’m good!