Figured this may be helpful to some ppl here, a simple, instantaneous way to remove chlorphyll from crude extract that is far preferable to winterization, as it targets only the chlorphyll and prevents the loss of terps/cannabinoids when winterizing an extract.
(edit: this is poorly worded, it isn’t an either/or thing. This tek is preferable to winterization at removing chlorophyll, but some waxes/fats will remain. Combining it with some gentle winterization afterwards - that is, less time and/or warmer temps - is probably the best technique)
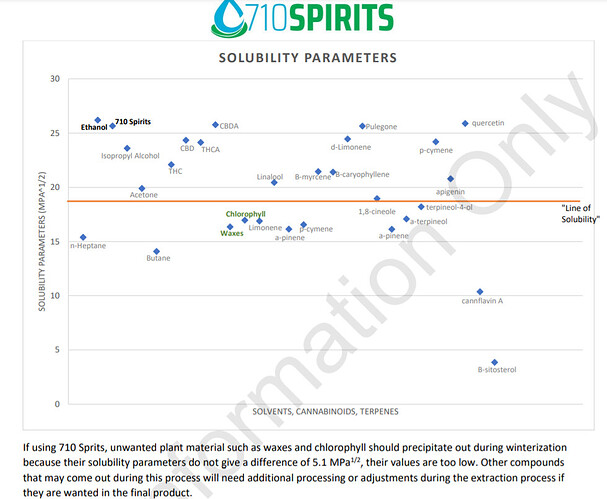
To start, it’s helpful to understand a bit about the chemistry and why winterization also removes some of the desirible compounds along with the waxes/fats/chlorophyll. Winterizing works by lower the solubilty of the solute in the given solvent. The waxes/fats that were soluble in your solvent at 20 C are much less so at -20, and so precipitates out during winterization. The problem with lower the solubility by temperature is that you cannot targe individual compounds…lower the temperature of a solvent lowers the solubility for ALL compounds in solution. Not equally, of course, and the compunds with the lower solubilities will precipitate out MORE than the ones with higher ones. Since chlorophyll/waxes generally have lower solubilities than other compunds present in crude, they’ll prcipitate out the most. But a loss of the desirible compunds is inevitable in winterization. This chart is a good way to visualize it. Basically, anything below the red line is going to make up the bulk of the precipitate.
Because the cannabinoids generally have higher solubility parameters than many of the terps, as well as the waxes/fats, their loss during winterization could be minimized by ensuring ideal solvent/solute ratios (but the ideal solvent amounts can be hard to figure out without doing a lot of complex chemistry math). That’s the good news. The bad news is, a lot of desirible terps have solubility parameters that are much closer to chlorphyll/waxes/fats and their losses can be a lot higher.
The chart doesn’t show methanol, but it’s solubility parameteris 36.2, compared to ethanols 26. This explains why methanol is a better extraction solvent, and a better winterization solvent as well…b/c it “holds” onto the cannabinoids better, so you’ll extract more than you would using an equal amount of ethanol. But it’s also got a “looser grip” on the fats/chlorophyll/waxes since it’s further away on the chart than those compunds, and will precipitate them out easier than an ethanol extraction. yes, it will also lose more of the terps in the 15-20 range than an ethanol extraction would, but if I have to decide between losing terps and losing cannbinoids, I choose the terps.
(incidentally, the entire report that that graph comes from is definitely worth reading in it’s entirety, you can find it here
In other words, winterization is a blunt instrument…it can remove chlorphyll and fats/waxes, but it will also remove desirible compunds as well. The way that I remove chlrophyll from my own extracts is a much more precise technique which targets only the chlorophyll. It’s also easy to do, and will remove the chlorophyll instantly. All you need are acetic acid (cleaning vinegar that you can get at any grocery/hardware store is fine) and copper oxide (can be had on Amazon for $15/lb or so).
First you need to create copper acetate, a blue water soluble salt. This can be done easily by boiling cleaning vinegar and stirring in copper oxide. The amounts aren’t really that important, I just eyeball it. I’ll just bring a few hundred ml of cleaning vinegar (10% acetic acid) to a boil, and add the copper oxide, put a magnetic stir bar in and leave it for an hour. what you’ll see is the vinegar has become a blue liquid. There may or may not be a black powder at the bottom. That’s unreacted copper oxide. If CO remains, all that means is you’ve reacted all the acetic acid in the vinegar into copper acetate, and no more remains to react the rest of the CO. You can either filter the solution to remove the unreacted copper oxide, or just add more vinegar until all the black powder has reacted. Usually, I’ll filter the solution with a bit of CO left in it so that I know I’ve reacted all the acid and I’m not carrying over any with my copper acetate, then just toss the unreacted oxide. You can keep it I guess, but it’s so cheap it just seems like a hassle to me. Then just boil away the blue solution and collect the blue salt that remains. This is copper acetate.
Then all you do to remove your chlorophyll is create a saturated solution of copper acetate in water and start adding it to your crude extract (I use methanol, but ethanol will work too). Measuring isn’t that important, you’re going ot be washing the extract with an organic solvent afterwards, which won’t pick up the copper acetate). You’ll instantly notice a lot of precipitation happening. This the chlorophyll. What’s happening is the copper acetate replaces the magnesium core of the chlorophyll with copper. Copper-based chlorophyll is much less soluble than the regular magnesium-based chlorophyll, and so it drops out of solution. Unlike winterization which drops the solubilities of ALL the compunds, copper removal only drops the solubility of the chlorophyll, which makes it a much more targeted technique than winterization. Let it sit for an hour or two, and then filter the extract. Once the extract is filtered, drop in some more copper acetate. If you see the same, goopy, amorphous precipitation, that means there’s more chlorophyll in the extract and repeat step 1. If instead you see a dark-bluish materiel forming on the bottom, that is simply the copper acetate coming out of the methanol solution. since copper acetate isn’t as soluble in alcohol as it is in water, when you drop a saturated solution of CA into an extract that doesn’t contain any more chlorophyll, the copper acetate has no where to go and just drops out of solution. This provides a handy way to know when you’re done. The copper chlorophyll precipitation is easily distingushed from the copper acetate precipitation, so you can be quite precise about exactly how much copper acetate is needed. When all is done, you should have a solution that is bluish in color, but much clearer than when you started.
At this stage, you can go two routes. You can winterize now to remove the remaining fats/waxes (remember, the copper acetate only targets chlorophyll, not the other undesiribles). If you do this, do a much more gentle winterization. don’t go below 0 C, and don’t leave it too long. Your extract is somewhat vulnerable now to a harsh winterization b/c now that the chlorophyll is gone, it’s easier to start cutting into your terps/cannabinoids. I just throw mine in the fridge for an hour or so and remove the samll bit of precipitate that sits on the bottom. Any more than this is unnecessary. The reason the bluish extract solution is clear post-chlorophyll removal is that chlorophyll makes up the bulk of the undesirible compunds, which is why further winterization is (in my opinion) more likely to cause more harm than good.
Or if you don’t want to purify any further, you can simply do a wash with Hexane (or chloroform, or DCM, or any immisicible organic solvent) and recover all the good stuff. You’ll notice that when you start the wash, your organic layer will be clear and your exrraction layer a dark blue. After the wash, organic layer will be a clear amber color, while the aqeous layer will be a light blue. That’s why you don’t need to be overly precise with adding the copper acetate, because it’s completely insoluble in any of the organic solvents and so it’s staying in the water layer. Before you do the wash though, I strongly recommend evaporating your alcohol off, preferably in a rotovap. If I had 1 L of post-chlorophyll removal extract, I would add about 200 ml of water to it (20%) and then gently rotovap the methanol out. While this isn’t crucial, the presence of the methanol will make the aqeous layer more soluble to the cannabolic acids, and so some will remain in the aqueous layer. If you don’t have a rotovap, or don’t want to remove the alcohol, I would dilute the solution to a 1:1 alcohol/h20 ratio before the wash. This will greatly reduce the solubility of the cannabiolic acids in the aqeous layer, and push all or most of them into the organic layer.
(side note about washing, due to the way solubility works at the chemistry level, i.e. Kpa/Ksp values and the like, it’s preferable to use smaller volumes of solvent relative to the crude solvent. Three washes of an equal volume is how it was taught in school. So if I have 1 L of crude extract, I’lll wash it with 1 L of Hexane, but rather than do one wash with 1 L exract/1 L hexane, I’ll do three washes of 1 L extract/0.333 L hexane)
Also, an added benefit to this method is that when the copper leaves the solution in the form of copper chlorophyll, the acetate ion it was attached too reverts back to acetic acid. Acetic acid reduces the solubility of THCA and CBDA in the aqeous solution even more, which will push more of those acids into the organic layer.
Pictures:
Acetic acid/Copper acetate solution, with unreacted copper oxide on the bottom
Filtered and evaporated copper acetate salt
Extract before adding copper acetate
5 minutes after adding copper acetate
After 1 hour
The black substance at the bottom (it’s actually a dark blue) is precipitated copper acetate. You can see it’s easily distinguished from the copper chlorophyll precipitate. The appearance of the dark blue acetate precipitate is used as an endpoint which tells us that all the available chlorophyll has been reacted.
Filtrate
final product (although needs another run through the filter as a little bit of the filtrate slipped through)
Organic wash to recover cannabinoids from the copper acetate solution. I’m using DCM here (whcih is why it’s on the bottom layer). this is before shaking, you can see the organic layer is clear (slightly yellowish, probably just from picking up minute amounts of solute on it’s way down to the basement)
After shaking for 20-30 seconds. Note the color change in both layers, the aqueous layer has become a beautiful sky blue after losing all the plant matter to the organic layer, which looks like a much more typical cannabis extract.
2nd wash (before shaking)
After shaking. Note the organic layer is more clear, and a lighter amber. This is because the first wash mostly removed the terps/THC (and fats that reminaed in the extract), and the second wash is primarily the cannabinoidic acids. I expect the organic layer on the third wash will come out basically the same color as it goes in, as there should be very little left to collect from the original crude extract