Yep you read that right !
We know by scaldiones papers that the molecule can exist
Hhc is way more stable than most cannabinoids so the the reagents could be accitic in nature
Who s got a hint for a good
Carboxilation reaction of HHC
The fact that it s a crystal at room temp is what intrigues me
And that HHC is now so damn cheap one cold use it as starting material instead of thc-a
Wouldn’t it require very high pressure CO2 + reagent? Recarboxylation would be super sweet if it didn’t have to be done at blow your face off pressures!
Nope I doubt any pressure is needed
Recaboxilation of cbd and thc can be done at atmospheric pressure so why not hhc
For cbd and thc recarboxilation rxn have to have certain ph limits to avoid isomerisation at the same time
But with hhc that’s less of an isseu so I think it must be feasible
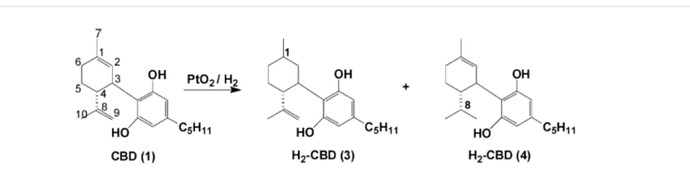
You do realize he made these through selective hydrogenation
Thc a > hhc a
Cbd a > hhcbd
He can control what bond hydrogenates on cbd, he says it in one of his long talks on hhc
If you’re looking to avoid thc a
Cbd a > hhcbd a > hhc a would do it too
There’s no reason it wouldn’t work, since your reaction conditions work with more reactive species. The only twist is the regioselectivity of your carboxylation might be hard to control since there’s no electronic or steric preference for either position.
You’re probably going to do this under basic conditions since the ring is very electron donating and CO2 is an electrophile. The phenolic alcohol will be deprotonated so there may be some hydrogen bonding that contributes to the selectivity that you want. My guess is that the OG carb position is preferred kinetically and they’re about the same thermodynamically. You can tell kinetically favored if you run at different temps (delta of 10c) and if you get less of one at a cold temp, it has a higher activation energy. If it is kinetically favored, run a lower rxn temp for longer and that will leverage the selectivity.
Another note, methanol is a common decarb catalyst, so it would likely also catalyze this reaction as well (in this case sodium method will be used).
You also could not give a shit about selectivity and I would have wasted my time typing this out…
![]()
That is indeed another option I didn’t realize
And good looking into
Since I am producing cbd-a at a shitload
Wonder what the racemic % would turn out to be ?
Usually it’s around 50/50
How do you expect to cyclize a fully hydrogenated CBDA molecule (hydrogenated at both double bonds)? You’d have hydrogenated the double bond required in the cyclization -_-
You hydrogenate the double bond outside of the ring before the one inside of the ring, and the bond outside the ring contains those precious electrons needed for the traditional cyclization of CBD to THC.
CBDA > dihydro CBDA > HHCA would not work electronically - you can’t cyclize fully hydrogenated CBDA like that.
That’s incorrect
He says this in his talk where he calls hhc a billion dollar molecule. You think he made hhcbd a synthetically? He didn’t
haha, okay.
well, you go right ahead and attempt to hydrogenate just the double bond inside of the ring and let me know how it goes.
My guess would be he protected the terminal alkene, taking advantage of different reactivity and then performed a hydrogenation followed by deprotection (note: i have not read the papers nor listened to the talk).
I dont need to attempt it, if I wanted to make it I’d do it lol
You sure are a spicy one when you’re proven wrong, you must have never actually done any research on what you’re saying or else you wouldn’t have said something impossible when it’s not
Typical trained chemist mentality
This is incorrect, if this was true 2h-cbd wouldn’t be reported as a mixture.
Directly from the patent
100mg cbd a > 86 mg hcbda
It’s all about the catalyst chose for hydrogenation
Edit: what they’re calling HCBDA is actually 4H-CBD-A
You can still partially hydrogenate cbd to get a mixture then separate it out if you really wanted to go cbd a > hhc a.
https://thecannabisindustry.org/tag/hydrogenated-cannabinoids/
I have done that reaction half a decade ago -_-
I created that mixture and separated them via chromatography and submitted for NMR in like…2018? You will require chromatography for a low yield and its hardly viable at scale. I don’t just think of a process as “can I do this in a vial and prove it can be done?” but more of “can I then scale this within reason and if so is it the smartest way to do this reaction compared to alternatives?” And it’s not, not really. Having step #2 be chromatography in a multi-step synthesis/purification is not good chemistry, its clunky and expensive. I mean, how else did you imagine this separation to be done? It would take some real insight into catalysis to optimize that selectivity, and it would likely still require chromatography.
By all means, you do you. Its a rabbit hole you’re welcome to throw time and resources at.
I keep hearing that recarboxylation can be done at atmospheric pressure but no one wants to spill the sauce. Is there source literature that I don’t know about cos there’s gotta be a better catalyst then magnesium methoxide
Magnesium methoxide is indeed flawed
Even under substantial pressure
MMC. Is a better option but still no great yield
Potasiumcarbonate is claimed by several but
Solvent volume is huge and yield not up to par as well
So I was thinking more into the lines of hcl
Potasium iodine and co2 sparge for it s pretty acid stable
The HHC is acid stable?
Yes quite depends on Wich acid and the concentration
WHO Can you recommend that I buy the best hhc from ? Can you help me with that