The cellulose is chiral.
Yes, you can make acetylHHC.
The cellulose is chiral.
Yes, you can make acetylHHC.
Would it retarded to try to separate HHC enantiomers on a column of microcrystalline cellulose?
What about methyl esters of cannabinoids? Not separation, but is it possible to make thc-methyl ester.
The stationary phase (a chiral media) is just more expensive to produce than typical normal or reverse phase silica. I mean, god forbid you need to do reverse phase chiral chromatography - that’s about as expensive as chromatography gets. No one chiral column will separate all chiral molecules on gods green earth - so you can spend a bit of money just finding the right column for what amounts to a difficult separation no matter how you look it at.
What’s the driving force for separation provided by chiral media? Attractive and repulsive forces between the chiral analyte and chiral media. Intramolecular forces like pi-pi interactions, dipole dipole, ionic interactions, hydrogen bonding that aren’t simply “differences in polarity of the molecule” that is usually what you rely on for achiral chromatographic separation. Its just a matter of finding a media that can exaggerates those intramolecular forces in such a way that one enantiomer is slightly more or less attracted to the stationary phase. I’m not a an analytical chemist - this was just sort of a rough explanation.
There is such thing as HHC acetate, and I can make it for you or anyone else if they so desire ![]()
No, but how would you get to the enantiomeric pair? You would have to use citronellal (racemic) and olivetol.
HHC from d8/d9 makes diasteromeric mixtures/pairs and while they ought to separate on a chiral phase, chirality of the stationary phase is not a necessity.
You can make a methyl ester of THCa, not THC.
I only suggest microcrystalline cellulose as a media because it’s exceedingly cheap compared to these prepackaged amylase columns folks have been mentioning.
Appreciate the responses.
@mitokid how many different forms of HHC are there?
@eyeworm if you get a contract for hhc acetate I’d gladly pay for a sample.
Yes, @eyeworm knows what’s up!
HHC has three chiral carbon centers so the number of structures are 2^3 = 8.
Four of those are cis at the 6a-10a junction.
And these are the four trans configured HHCs:
The little black dot represents a hydrogen atom pointing towards you.
A and B is what you get from hydrogenation with chirality at 6a and 10a already defined by Nature. A and D is what you get when using the (racemic) citronellal and olivetol route.
Sorry to ask basic shit but cis and trans refer to chirality?
What does the 6a-10a refer to?
Also sorry to @eyeworm for cluttering up your thread with my questions and my bitching (further up).
The 6a and 10a carbons are the ones at the junction of the two non-aromatic rings. In the cis structures, the hydrogens are on the “same” side. In the trans structures, they are on opposite sides of the junction.
And in d6a(10a) there’s a double bond between 6a and 10a so there’s no longer a notion of cis or trans.
So the red circles both denote the “6a” position and the “10a” position? Red is the double bond?
The blue circles are the point that denotes chirality or rather its the cis vs the trans? Idk what the difference is.
Or is it the black dot that denotes the hydrogen?
Consider this. On THC (d9 or d8) these are already two chiral centers - we just don’t talk about it much since they’re always right handed (R) because that’s how the plant makes it. The CBD used for making d8 is all (RR), the d8 from that CBD is all (RR) and the d9 on the plant naturally is all (RR). Nature is good at making the right stereoisomers every time, and these particular reactions are not touching these chiral centers.
But when you hydrogenate, you form a new third chiral center. And because of the hydrogenation mechanism you can produce either S or R is this center. For convenience I’m going to butcher some shorthand - lets say you started with your everyday d8-THC - two chiral centers, both R. Lets call it (RR)-d8-THC. Now you hydrogenate it, add a chiral center, and you have a mixture of (RRR)-HHC and (RRS)-HHC. Those are not enantiomers - enantiomers are stereoisomers which you cannot superimpose and are mirror images of each other (meaning all available chiral centers are opposites of each other). Stereoisomers that are not mirror images are diastereomers - and they require multiple chiral centers like we have here.
(RRR)-HHC and (SSS)-HHC are enantiomers because all three chiral enters are opposite - or mirror images of each other. You wouldn’t be making (SSS)-HHC in this reaction because the original two chiral centers present on the delta 8 starting material are already R and R because that’s how the plant made the molecule, and hydrogenation doesn’t change this.
(RRR)-HHC and (RRS)-HHC (which is what we’re making when we make HHC via delta 8) are diastereomers because they are indeed stereoisomers, but they are not mirror images at all three chiral centers. Because only one of the chiral centers differs between the two molecules, they are not enantiomers. And you can separate diastereomers without chiral chromatography, whereas true enantiomers require chiral chromatography.
The maximum theoretical number of stereoisomers is 2 to the N power. 2 represents the R and S possible orientations, and N represents the number of stereocenters (number of chiral carbons). So 2 to the third is 8 possible stereoisomers of HHC. However, if you’re starting from delta 9 and two of the chiral centers are fixed - you are only creating 2 to the first power stereoisomers (the RRR and RRS diastereomers).
edit: I drank beer and lost focus while typing, fixed some stuff.
This is the one you want, all-R HHC:
At carbons 9 and 10a, there’s no need to indicate the fourth substituent, the hydrogen atom, since the three other substituents “directionality” is indicated.
At 6a, the three bonds around that center is considered to be in the plane of the paper and the fourth substituent can either be below or above the 2D representation. The solid black dot means that hydrogen is above the “plane”, closer to you, the observer.
I’ll have to digest this, thank you.
Cannot superimpose.
What determines the # position on a molecule?
ya I slipped up, I blame the good times.
Nomenclature rules by governing bodies of chemistry. Cannabinoids have had two, concurrently used numbering systems.
Nowadays, it is all d8 or d9. Back when Mechoulam was in full swing, those structure were called d1(6) and d1, respectively.
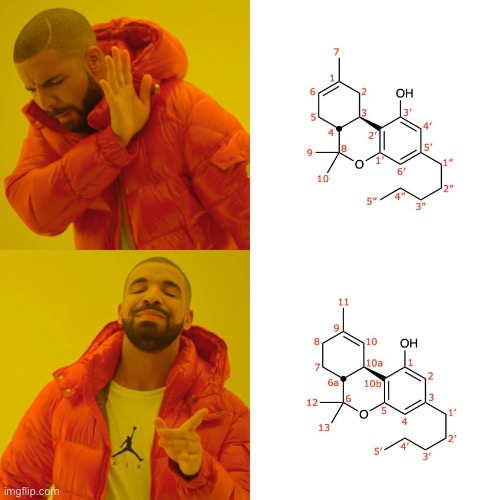
Yeah, I noticed old papers refer to cannabinoids as different molecules or at least slightly different names, which is why I asked.
So the delta 1 referred to in this paper refers to delta 9? Most likely?