If it’s not degradation, it’s just the lab testing. If you ask them for a chromatogram and DM it here or instagram I’ll be able to tell you a lot more about it.
Asked for the chromatograms, so should have that soon. Thanks again for the help!
I would like to see the original chromatograms. This is extremely important, if they injected lower amounts during the first run they could have “below calibration” value then if they injected more in the second run they could see the CBDA peak within the calibration range.
One more thing, did they use same HPLC column? If you have retain samples, you can ask them to re-run these two samples in a raw.
This is what they sent:
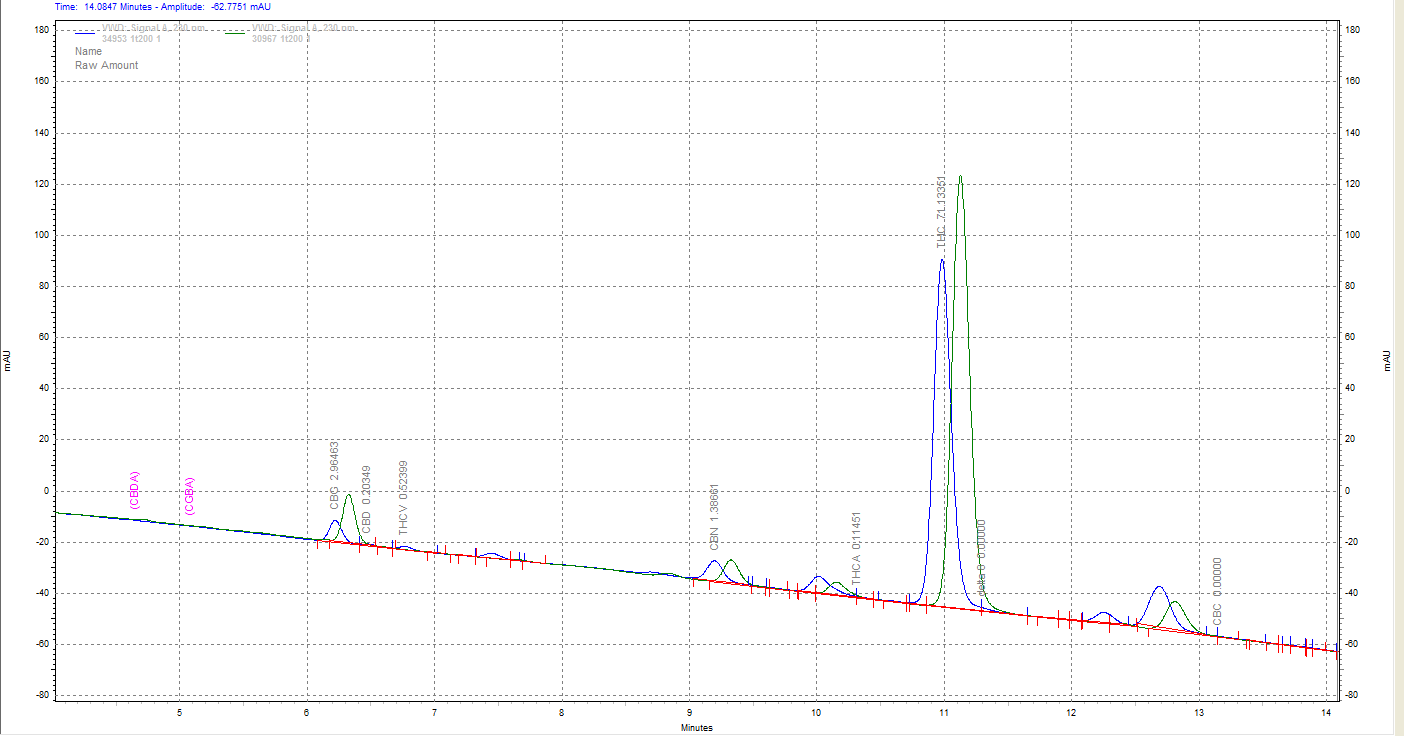
1st pass distillate in green overlayed with the low potency 2nd pass in blue. The unlabeled peak at ~12.7 min is larger in the 2nd pass. Anyone know what this peak corresponds to? d10 elutes near CBC I think? And the peak at 12.25 min that is present in the blue 2nd pass but appears absent in the 1st pass?
@tetramethylsilane Not sure if this applies to the peak? ![]()
Thank you! Interesting if it is d10. Is it common to see d10 in samples with no d8? I’ve only ever seen what I figured to be d10 in high d8 samples.
The distillate in question here had no processing other than 1st pass distillation, no adsorbents in flask, or anything else that would normally make me think of isomerization.
You did heat it up a second time…
Given you** can isomerize to D10 without catalysts or additives on a first pass, it is not unreasonable to suggest “over heating”.
** not certain about “you”. Pretty sure I’ve done it…
Ah, I didn’t realize there could be significant isomerization from heat alone. I thought an acidic environment was required.
Well shit. While I have not required excessively hot temps, my distillations have been taking ridiculously long - see:
This is a degradation issue, those peaks represent more mass than you would think. If you want them quantified and can get a sample to me I’d be happy to do it.
We hope that very soon at least two of these peaks will be common place in other labs. My company (VSW Remediation) is presenting at the emerald conference on isolation of two of those peaks and NMR data from those peaks. It will end with a recommendation that all labs test for them even if not required by their regulatory agencies. They’re in everything and all you’ll find is crickets chirping when you look into their effects and safety.
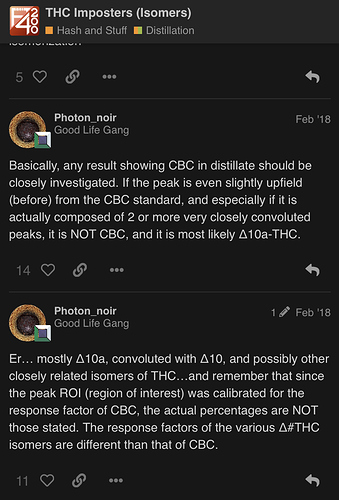
What do you think they are? d10 / d10a?
I see that the lab has an HPLC problem - the retention times are not stable and they have some baseline drift (negative). I would ask them to inject a blank (just solvent without your sample), then your sample number 1, then your sample number 2, and a blank again. Can they make it for you? We can see some minor peaks, but the origin of the peaks is not clear.
Yes. Any other isomerization (like D8) is common with the D6a and D10 isomers. These are the super-shorthand names… they are technically called D6a,10a-THC and D10,10a-THC, referring to the location of the double bond.
Yes, it can happen spontaneously (no catalyst) with high heat history, i.e. long time or repeated heating. Catalysts just help minimize the activation energy of the reaction. If you exceed the necessary activation energy without catalyst, the reaction still happens.
This is something I’ve experienced as well. Which is why I collect all my client’s product in Food Grade 5 gallon buckets and homogenize the entire batch in a reactor before bottling and collecting samples for final COA testing. Of course I collect samples randomly throughout the process for in house analytics but I never send out for third party unless representative of the entire batch.