I have been looking to determine a function for the change in the heat of vaporization.
This is so I can estimate the pressure-temperature boiling point’s. I had two questions
about this; is there data that I am just not finding, or will i need to do my own experiment?
Also, since I am planning on using this too help with operating conditions for our centrifugal
distillation equipment, will this data even help me with that, since we are dealing with mole
fractions of the substance, instead of the pure substance?
NOTE: all that is required for me to do so is some data points on boiling points of a given cannabinoid at different pressure, the more the better. I made a rough estimate of 3 different boiling points and the result was up too a 20% error from know values. The more data the better the function’s fit.
Two reliable data points for D9 THC are that it “boils” at
200C at 20 microns (Pubchem)
127C at one micron (observed by me)
From these two you can extrapolate the enthralpy of evaporation in a crude fashion and create the curve you are after. These represent pure compound observations. I originally computed the curve myself at 120C on the low end but have since revised this based on further observation. I have multiple videos demonstrating boiling temps needed under various configurations. I have not replotted the curve since revising the low temp but it would simply be a plot based on the numbers just given. I hope this helps.
That is essential what i did, but i am noticing that the trend I get is not the best (R^2 < 0.92) for an exponential or poly function. I just assumed with more data comes more accuracy. I used this data: 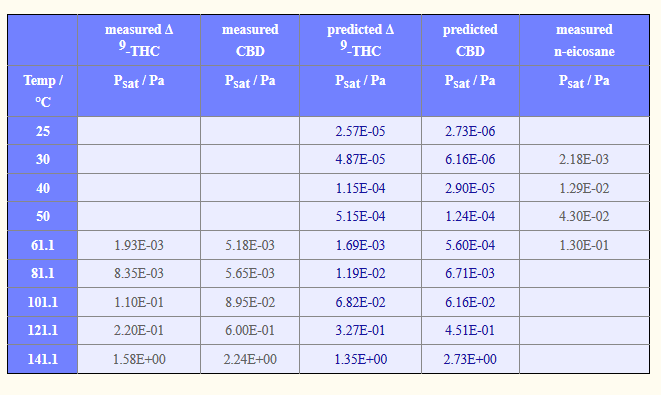
For a follow up question, When distilling did you notice a large change between the pure components vaporization temperature and the one mixed with the crude?
edit: Also would you mind linking me to those videos you made?
Here is a link to some that tend to show data.
https://vimeo.com/album/5262404
@grassfedlabs,
This was the discussion I was referring to.
I hope you find this community resourceful!
Welcome new user!
You can use Clausius Clapyron, and predict it for a pure substance…using the enthalpies of vaporization for cannabinoids. The latest seems to be 93 kj/mol for THC and 120 kj/mol for CBD. Thats what I did for terpenes and cannabinoids, and I developed a good terp strip procedure using my “ball park” predictions.
you are the best, thanks