I’ve noticed in threads over the last year or so that a lot of folks either have no experience with or want to learn more about analytical chemistry for themselves or their career. I didn’t see really any threads on the subject but wanted to create a space for the discussion.
I’ll start with some overview. A lot of you guys are used to the concept of chromatography as a sample prep or purification method. But the concept of Spectrophotometry at large for analysis is often new territory.
In chemical analysis, at least as it relates to cannabis R&D, we more or less are seeking to answer two questions. What it is it? How much of it is there?
We answer these questions by using one of the most essential components of the universe, light.
Spectrophotometry, what is it?
Wikipedia defines it as “In chemistry, spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength.[2] It is more specific than the general term electromagnetic spectroscopy in that spectrophotometry deals with visible light, near-ultraviolet, and near-infrared, but does not cover time-resolved spectroscopic techniques.”
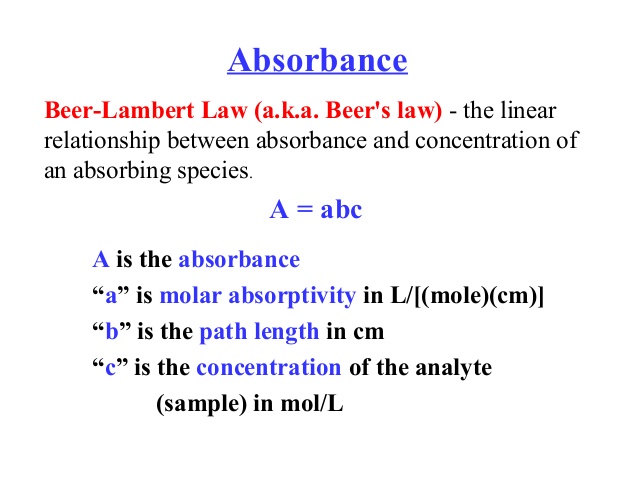
To start understanding this concept better, it would be good to understand Beer’s Law.
“Beer’s Law is an equation that relates the attenuation of light to properties of a material. The law states the concentration of a chemical is directly proportional to the absorbance of a solution. The relation may be used to determine the concentration of a chemical species in a solution using a colorimeter or spectrophotometer”
There are many instruments and techniques used to do this, but I will try to keep the scope of this limited to instruments generally used in our industry.
When talking about the instruments used to conduct these applications, we should probably split them into two overlapping, but somewhat individual concepts of Structural Elucidation and Constituent Quantification.
Structural Elucidation is the process used to determine the chemical structure of a compound. The instrument most notably associated with this is NMR (Nuclear Magnetic Resonance) spectroscopy.
“NMR is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups.”
Constituent Quantification is the process of determining what compounds are present in a sample and what percentage of the sample they are.
In our industry, and many others, the most notable instruments used for this are TLC, HPLC, LCMS, and GCFID/MS
"Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures.[1] Thin-layer chromatography is performed on a sheet of glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. This layer of adsorbent is known as the stationary phase.
“High-performance liquid chromatography ( HPLC ; formerly referred to as high-pressure liquid chromatography ) is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column”
“Mass spectrometry ( MS ) is an analytical technique that measures the mass-to-charge ratio of ions. The results are typically presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.”
“Liquid chromatography–mass spectrometry ( LC-MS ) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography (or HPLC) with the mass analysis capabilities of mass spectrometry (MS).”
Gas chromatography–mass spectrometry ( GC-MS ) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample.[1]"
The structural elucidation of cannabinoids in particular began way back in 1932 with CBN, followed by CBD in 1963, and D9THC in 1964. The other major and minor cannabinoids began to be isolated and elucidated in the 1970s. Structural elucidation is a very important piece of understanding molecules, we can better understand the compounds at hand this way, and is a large component of how we know the molecular structures that even cannabis amateurs are familiar with today. However, chemical structure is not necessarily required to conduct chemical analysis for a laboratory. We can also use the information from structural elucidation to predict what the biological action of a compound may be and how to approach purification and extraction, among many other things.
By making use of constituent quantitative analysis in a company R&D/QA lab we can make more effective decisions on the product development process, better protect consumers, and meet compliance standards.
Citations:
Edits: added basic information