It has been awhile since we’ve posted and there have been a lot of new developments here at KCA.
One issue worth posting about is the problem around false negatives for the presence of D9-THC in crystal-resistant distillates or THC remediated materials. Many clients have had materials stopped by international customs offices and we’ve had our results challenged by laboratories using a non-specific detector, such as HPLC-PDA (or -DAD).
The lack of specificity on the commonly used reverse phase HPLC methods in the industry are leading to testing labs ignoring the need for further work analyzing these materials to achieve better resolution.
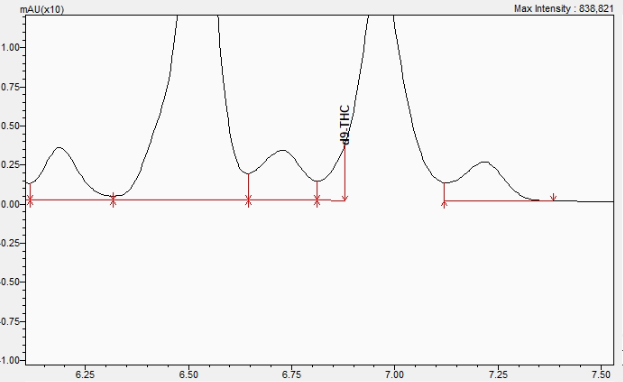
As seen on the HPLC-PDA chromatogram below, a retention time shift (compared to D9-THC) and review of the UV spectrum of the peak eluting just before 7 minutes will lead to identification that the peak is not D9-THC. What we’ve come to find out is that the D9-THC is coeluting and fronting this unknown peak. See how we’ve integrated the D9-THC:
We’ve run similar samples on LC-MS/MS and have the same issue with coelution.
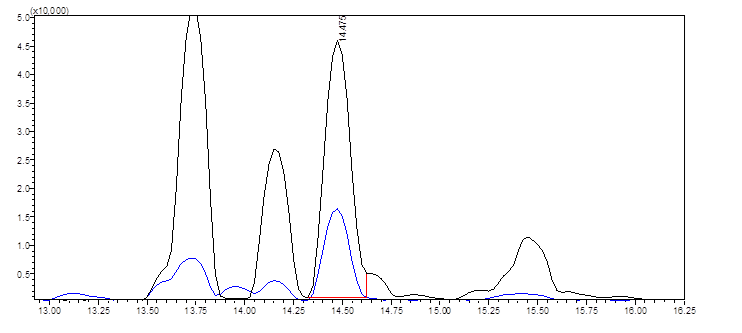
One of KCA’s lead scientists has been hard at work developing a method to achieve better resolution (still under development). The following shows the TMS derivative of D9-THC eluting at 14.475 minutes for the same sample. When quantified we obtain a value similar to the integration performed on HPLC. We’re avoiding most of the coeluting mess on HPLC and we’re able to identify and quantify D9-THC with greater confidence. This is the same sample as above:
Both false negatives and false positives for D9-THC can do damage to a company’s reputation and ability to import/export, so I urge everyone to avoid trusting only RP-HPLC analysis of CRDs and remediated CBD materials. We’ve seen that some labs will call the D9-THC as ND and others may call the entire coeluting peak on HPLC as D9-THC. If you need truly compliant or truly ND CBD distillate then have a laboratory with derivatization and mass spec capabilities analyze your material. Ask your testing laboratory for chromatograms and ask them to identify the retention time of D9-THC to check the baseline. The data belong to you.
18 Likes
I like your GC method for way more than any derivitization method.
1 Like
D9-THC can still have issues with coelution on our existing GCMS method. The derivatization helps separate it from the unknowns.
Results from some of these remediation methods make me wonder if something is being added to mask the D9-THC rather than truly remediate it. While on the other hand the new method allows us to confirm samples that are indeed remediated.
1 Like
Any merit to the claims that gc itself is causing additional conversions due to heat?
Most all the dev rxns I have seen do some variety of silylation reagents.
How do you verify the completeness of your dev rxn? Id make sure the canna sample is your limiting reagent, but then you have to think of possible side reactions with your samples. what if your side rxns are outcompeteing your target rxns
doing this with isolates / distillates is one thing, but when trying on the extremely wide range of flower or infused products, it seems like an impossible goal
1 Like
@pdxcanna negligible and derivatization helps control for those changes and the derivatized acids do not decarboxylate, which is the main reason derivatization methods have been introduced into this industry.
@ShuckleBerryFinn yes, we’re aware and we’re keeping an eye on it during method development.
3 Likes
not really, you just throw excessive molar equivalents at the rxn and blow it out the water.
How do you ensure something will be the limiting reagent? Throw an excessive shit ton of every other reagent at it.
1 Like
Do you expect a shit ton of excess on other reagents to have no effect on your analysis ?
youd want to run (at bare minimum) your controls with your dev reaction in the same proportions as you react your samples.
if your goal is to derivatize everything in there without a doubt, then no I do not expect that using excess reagent would be problematic - in fact, its exactly how it should be approached. You do a workup before analyzing to wash away unreacted reagent, its pretty straightforward.
The reactions used to put a protecting group somewhere on a molecule are usually pretty selective and high fidelity, because if they weren’t selective then they would fail to be useful. If you choose the right protecting group and conditions, the excess reagent in many cases is just left unreacted. For instance acetylation of an alcohol group - if you acetylate all of the available alcohol groups, any unreacted base and acetic anhydride just get washed away in the workup. They don’t just wreak havoc doing random chemistry - because again, the reactions we’re invoking are selective towards acting on a specific functional group.
Look through this list of protecting group syntheses. You’ll notice just about all yields are effectively pushing 100% - again, because these types of reactions are necessarily highly selective towards specific functional groups.
2 Likes
Use of appropriate combination of derivatization agent and solvent also helps in pushing reactions to the max.
The remediation by simple thermal degradation produces two compounds which are impossible to separate completely from the remaining 0.0x% of d9 by normal GC, unless perhaps using an excessively long column, or derivatization as you show here.
3 Likes
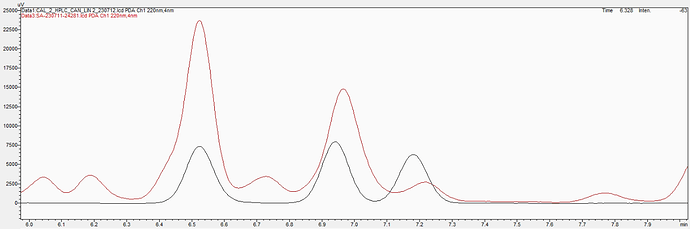
Here is more from the example sample in the original post.
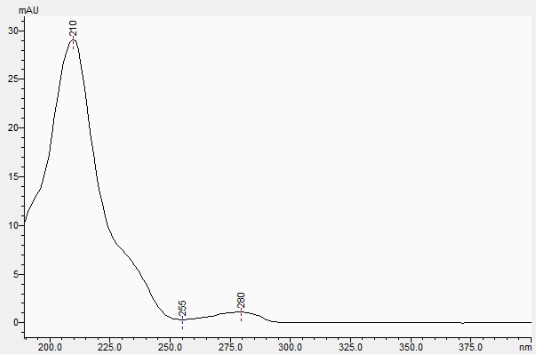
This HPLC-PDA chromatogram shows the standard (black trace) and the sample (brown trace) and you can see how the D9-THC would front the peak in the sample if it could be detected.
The absorbance spectra also hides the D9-THC.
Standard
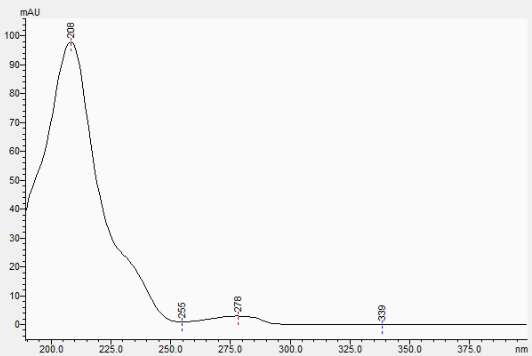
Sample
1 Like